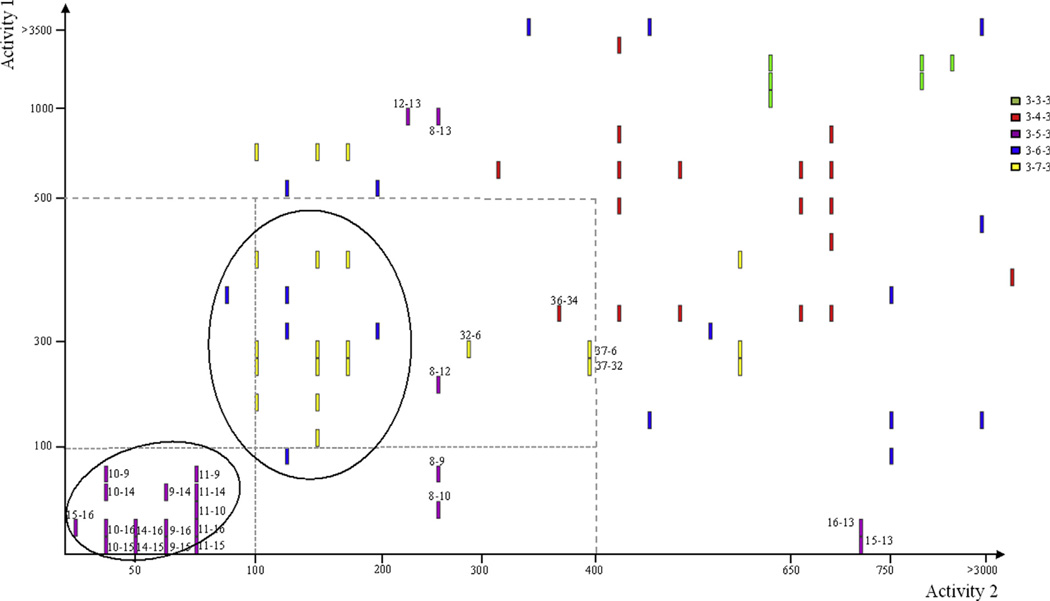
Figure 6.
Structure activity landscape analysis for the symmetrically substituted (bis)urea- and (bis)thiourea polyamines. Pairwise activity to structural feature analysis was performed with Activity Cliff Analysis (Osiris DataWarrior V 3.12.1) on the complete series from Figure 5 at a stringency of 80% similarly in structural features. Cluster analysis based on backbone structural features. In all cases, two structurally similar isosteres were compared and a single bar acts as identifier of such correlations based on the respective activities of the two isosteres as indicated on the two axes (decreasing potency from crosspoint). Activity values are based on the In vitro IC50 values in nM (asexual P. falciparum parasites 3D7). Isostere pairs are identified numerically based on compound numbers in Figure 5 for example, 15–16 with activity 1-activity 2 desciptors.