TABLE 1.
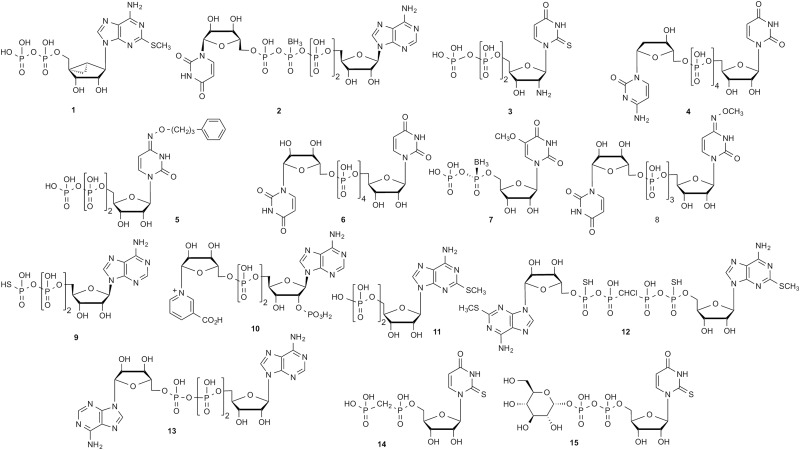
Representative examples of different types of synthetic or natural ligands (agonists, unless noted), either mononucleotide or dinucleotide (or nucleotide sugar), for each of the P2YRs
The potency (nM) was measured in functional assays at the human P2YRs.
 | |||
|---|---|---|---|
| P2YR |
Synthetic Agonist (Mononucleotide), Potency |
Bifunctional Ligand, Potency |
References |
| nM | |||
| P2Y1 | 1, MRS2365, 0.4 | 2, Up4(β-B)A, A isomer, 500 | Houston et al., 2008; Yelovitch et al., 2012 |
| P2Y2 | 3, MRS2698, 8.0 | 4, INS37217, 220 | Houston et al., 2008; Yerxa et al., 2002 |
| P2Y4 | 5, MRS4062, 26 | 6, INS365 (Up4U)a, 130 | Ko et al., 2008; Maruoka et al., 2011 |
| P2Y6 | 7, 5-OMe-UDPαB, 8 | 8, MRS2957, 12 | Maruoka et al., 2010; Haas et al., 2014 |
| P2Y11 | 9, ATP-γ-S, 24,000 | 10, NAADP, 64,000 | Djerada and Millart, 2013 |
| P2Y12 | 11, 2MeSADPb, 5 | 12, compound 17 (R/S)c, 13 | Zhang et al., 2002; Yanachkov and Wright, 2010 |
| P2Y13 | 11, 2MeSADPb, 19 | 13, Ap3A, 72 | Zhang et al., 2002 |
| P2Y14 | 14, MRS2905, 2.0 | 15, MRS2690, 70 | Das et al., 2010 |
NAADP, nicotinic acid adenine dinucleotide phosphate.
INS365 also activates P2Y2R (EC50 = 210 nM).
2MeSADP activates P2Y1R (EC50 = 6.6 nM), P2Y12R, and P2Y13R (also used as a high affinity 3H- or 33P-radioligand) (Takasaki et al., 2001).
Antagonist.
