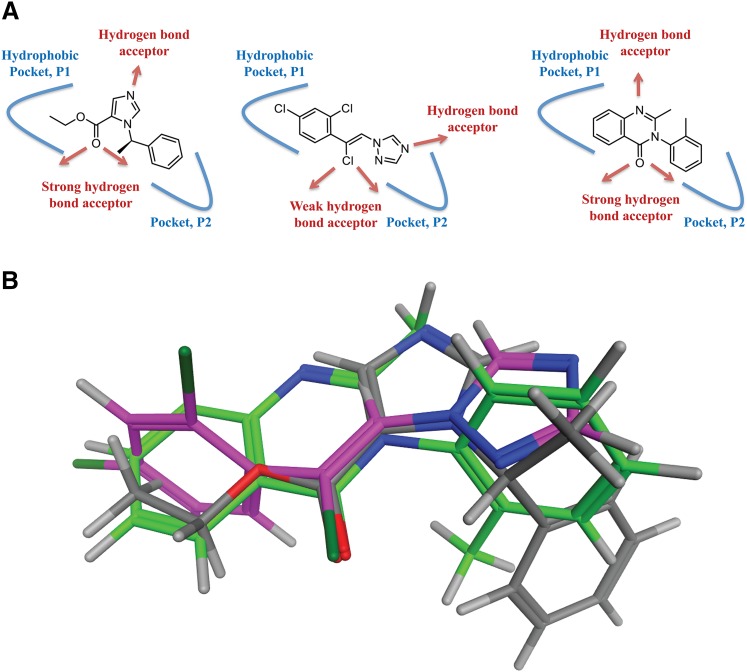
Fig. 6.
The putative shared binding mode of etomidate, loreclezole, and methaqualone. (A) Illustration of the structural similarities between etomidate (left), loreclezole (middle), and methaqualone (right). The putative pockets P1 and P2 are given in blue, and the hydrogen bond acceptors in the compounds are indicated with red arrows. (B) Superimposition of low-energy conformations of etomidate (type code), loreclezole (pink), and methaqualone (green) by fitting the carbonyl groups of etomidate and methaqualone and the vinylogous chlorine of loreclezole.