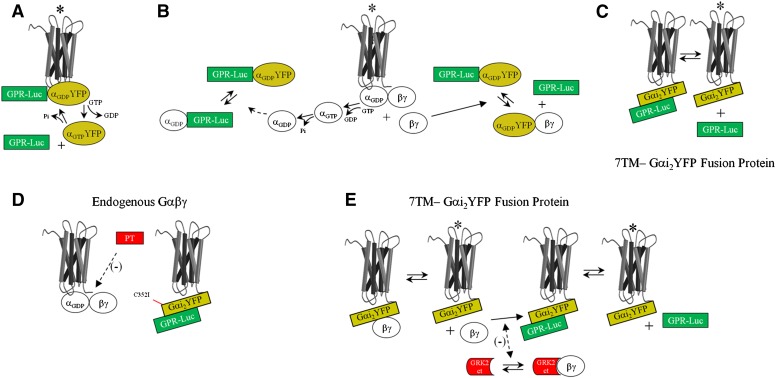
Fig. 1.
Schematic representation of hypotheses regarding regulation of GαiGPR by a 7TM receptor. Agonist-induced reductions in GαiYFP–GPR-Rluc BRET may reflect the following different scenarios. (A) Direct coupling of the receptor to the GαiGPR module, which is regulated by agonist-induced nucleotide exchange on GαiYFP. (B) Competitive inhibition of Gαi binding to GPR proteins by endogenous Gα or Gβγ liberated subsequent to receptor coupling to endogenous Gαβγ. (C) Agonist-induced regulation of the interaction of GPR proteins with an α2AAR-Gαi2YFP fusion protein. (D) To determine the influence of endogenous Gαβγ on basal and agonist-induced regulation of α2AAR-Gαi2YFP:GPR-Rluc BRET, Cys352 in Gαi2 was mutated to Ile (C352I), rendering the tethered Gαi2YFP insensitive to pertussis toxin. Receptor coupling to endogenous Gαβγ could then be blocked by cell treatment with PT (Burt et al., 1998). (E) Endogenous Gβγ subunits may also engage the α2AAR-Gαi2YFP fusion protein (Burt et al., 1998). GRK2-CT was expressed as a scavenger for free Gβγ released subsequent to receptor activation of an α2AAR-Gαi2YFP fusion protein complexed with endogenous Gβγ. *Receptor activated by agonist.