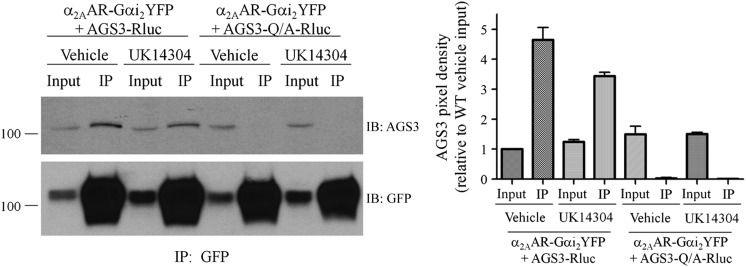
Fig. 4.
Coimmunoprecipitation of AGS3 with the 7TM receptor–Gαi2 fusion protein is regulated by agonist. (Left panel) HEK293 cells expressing α2AAR-Gαi2YFP and AGS3Rluc for 24 hours were treated with α2AR agonist UK14304 at a final concentration of 10 μM or with vehicle (Tyrode’s solution) for 5 minutes at room temperature, as described in Materials and Methods. Cell pellets were sonicated in immunoprecipitation (IP) buffer and cell membranes extracted with 2% n-dodecyl-β-d-maltoside followed by immunoprecipitation with anti–GFP-Sepharose overnight at 4°C. Immunoprecipitates were washed and resolved by SDS-PAGE and immunoblotted (IB) with AGS3 antisera (upper panel) followed by stripping of the blots and reprobing with GFP antisera (lower panel) as described in Materials and Methods. “Input” represents 1/20 of the total volume of cellular lysate taken prior to immunoprecipitation. AGS3-Q/A refers to the mutation of a conserved glutamine residue in each of the GPR motifs to alanine, which renders them incapable of binding Gαi. (Right panel) Densitometric analysis from the means of two independent immunoprecipitation experiments as shown in the left panel, with pixel density set relative to the AGS3–wild type (WT) vehicle-treated input.