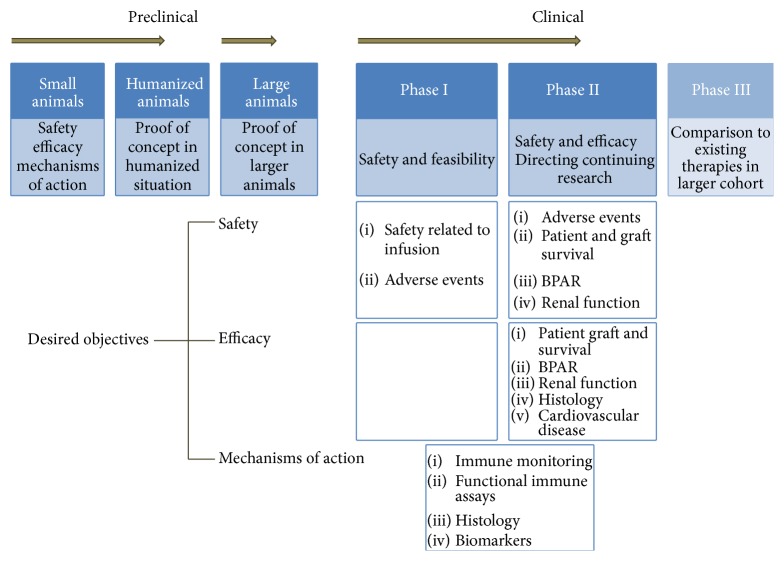
Figure 1.
Desired objectives in clinical studies with MSCs in renal transplantation. Preclinical studies with MSC in the transplant setting start with small animals to investigate safety, efficacy, and mechanisms of actions. Then studies move on to prove the concept in humanized animals and larger animals. Human phase I studies address safety and feasibility in a low number of patients and determine the direction of further research. Phase II studies focus on both safety and efficacy parameters, which include patient and graft survival, BPAR, renal function, histology, and cardiovascular disease. Surrogate markers, such as immune monitoring and functional immune assays, are used to determine mechanisms of action. BPAR: biopsy proven acute rejection.