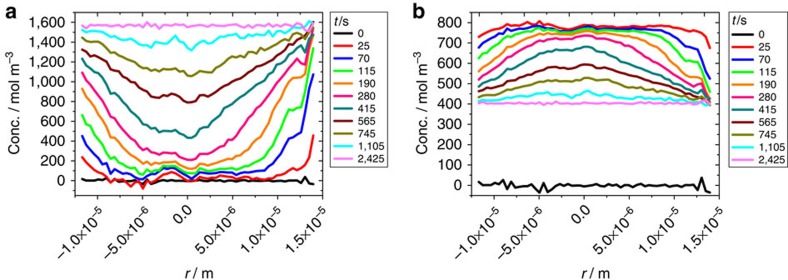
Figure 2. Single-component profiles during uptake of C2H6–CO2 mixtures.
(a) Evolution with time of the concentration profiles for ethane (the slow component), after the initially activated crystal of zeolite ZSM-58 has been brought into contact with a C2H6–CO2 mixture with partial pressures of each component of 200 mbar in the surrounding atmosphere. With the ethane front penetrating the system, CO2 is partially desorbed as shown in b. Finally both components reach uniform equilibrium concentrations determined by their partial pressures.