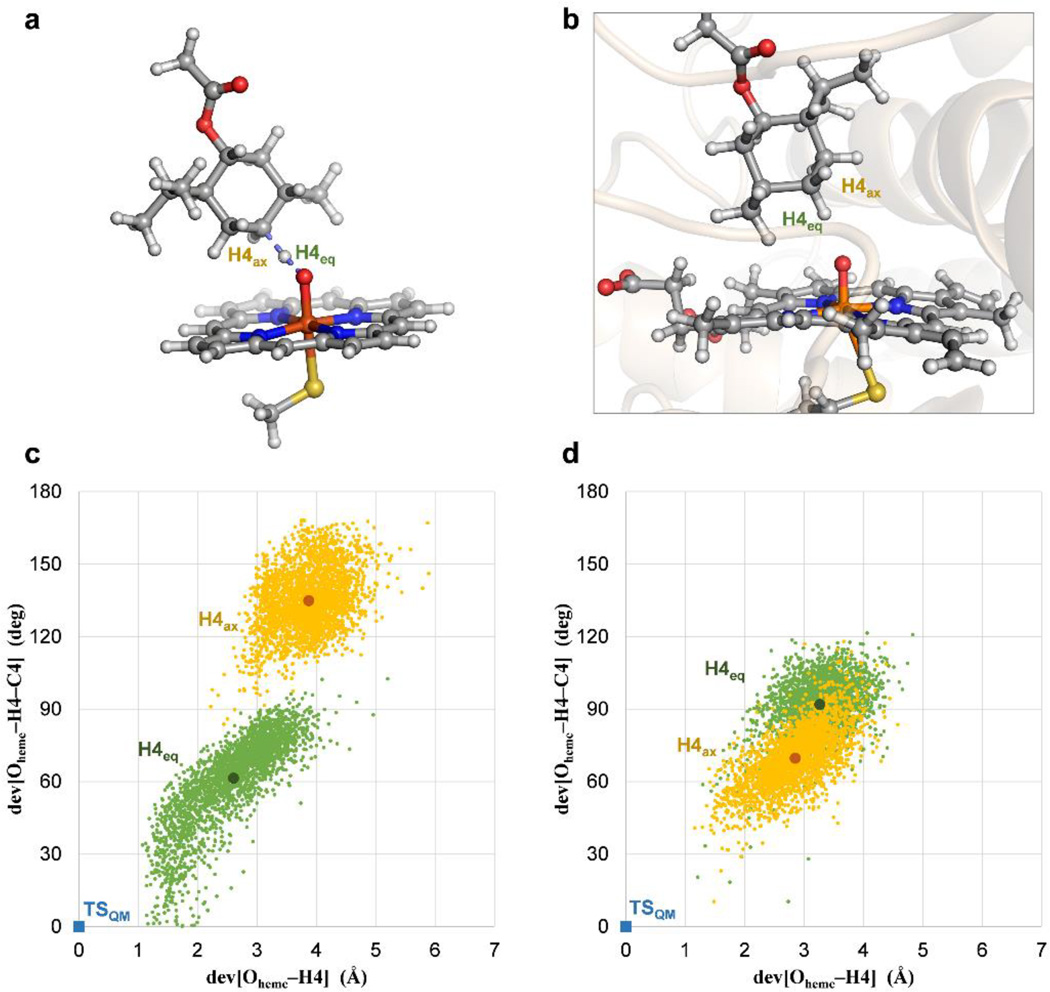
Figure 5. Stereoselectivity in the C4–H abstraction catalyzed by PikC.
(a) Quantum mechanically (QM) optimized transition for hydrogen abstraction of H4eq in (−)-menthol (see Supplementary Fig. 2). (b) Representative snapshot of a MD trajectory of PikCD50N bound to 25 showing the substrate in close proximity to the catalytic heme iron-oxo species (Compound I). (c) and (d) Orientation of H4eq (in green) and H4ax (in orange) of (−)-menthol (c) and (+)-menthol (d) relative to the iron-heme oxo group in PikCD50N, derived from 0.5 µs MD trajectories. Each point represents a simulation snapshot. Deviations of the distances (x axis) and angles (y axis) from the quantum mechanically optimized transition structure (in blue) with a hypothetical model heme catalyst are shown. The greater separation between the two sets of points (H4eq and H4ax) in (−)-menthol is interpreted as a potential higher diastereoselectiviy in the associated transition states, and thus the hydroxylation reaction.