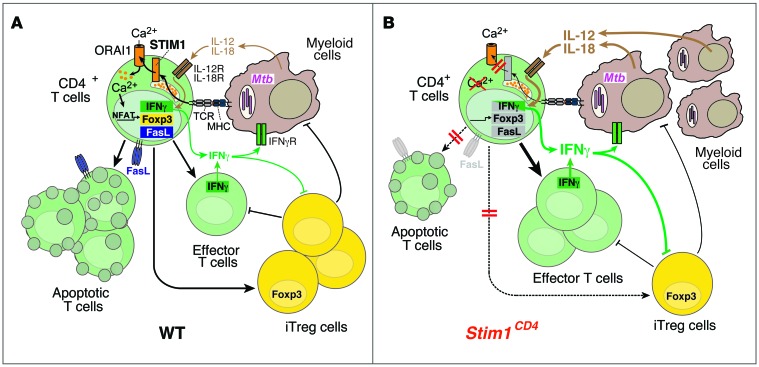
Figure 8. The role of STIM1 in chronic Mtb infection.
(A) In WT mice, Mtb-infected macrophages/DCs present mycobacterial antigens via MHC class II to CD4+ T cells, resulting in STIM1 activation, its binding to ORAI1, and Ca2+ influx (SOCE). SOCE activates NFAT and other Ca2+-dependent transcription factors, which regulate Fasl, Foxp3, and Ifng gene expression. FasL mediates AICD in T cells following repeated TCR stimulation, thereby preventing lymphoproliferation in chronic infection. FOXP3 expression is required for the differentiation of iTregs that suppress effector T cells and potentially myeloid cells. IFN-γ production after TCR, but not IL-12/IL-18, stimulation is dependent on STIM1/SOCE. IFN-γ secreted by effector T cells helps to contain Mtb infection and to recruit myeloid cells. (B) In STIM1-deficient mice, ORAI1 activation and SOCE are impaired, resulting in reduced expression of Fasl, Foxp3, and Ifng and other genes. Impaired AICD results in accumulation of effector T cells in Mtb-infected mice. Despite impaired TCR-dependent IFN-γ production, STIM1-deficient T cells respond to IL-12/IL-18 and secrete more IFN-γ during chronic Mtb infection, promoting the recruitment of myeloid cells that can be infected with Mtb. Higher bacterial burdens result in increased secretion of IL-12/IL-18 and perpetuation of pulmonary inflammation. Secretion of IFN-γ by T cells and IL-12 by myeloid cells inhibits differentiation of iTregs, adding to the STIM1-dependent, T cell–intrinsic defect in iTreg development. Together, these defects result in pulmonary hyperinflammation/consolidation, loss of respiratory function, and premature death of Stim1CD4 mice.