FIG 8.
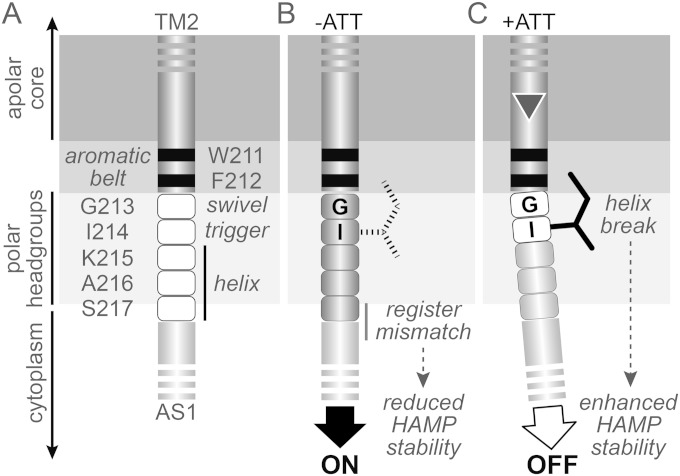
Mechanistic model of transmembrane signaling in Tsr. All panels show residues at the TM2-AS1 junction of one Tsr subunit and the interfacial region of the membrane, shaded from light gray (polar) to dark gray (apolar). This membrane location is based on studies of the TM2 segment in the Trg chemoreceptor (45). Similar experiments with Tar-TM2 (46) suggested that the second and third control cable residues in that receptor might lie within the transition zone between the apolar core and polar headgroups. (A) Signaling roles of control cable residues (white fill). The C terminus of TM2 and the N terminus of AS1 are assumed to be α-helices (dark gray and light gray cylinders, respectively). Three of the control cable residues (K215, A216, and S217) are proposed to favor an α-helical secondary structure in both signaling states, whereas stimulus input modulates the helix potential of G213 and I214. (B) The ON state. All five residues of the control cable adopt a helical secondary structure, which propagates the TM2 helix register toward AS1 and destabilizes the HAMP bundle. The I214 side chain (broken lines) plays no critical structural role in this signaling state. (C) The OFF state. Inward piston displacement (triangle) of TM2 induces a break or kink at G213 of the control cable, alleviating the register mismatch between the TM2 and AS1 helices and stabilizing the HAMP bundle. Interaction of the I214 side chain with the membrane interfacial region promotes this structural change.