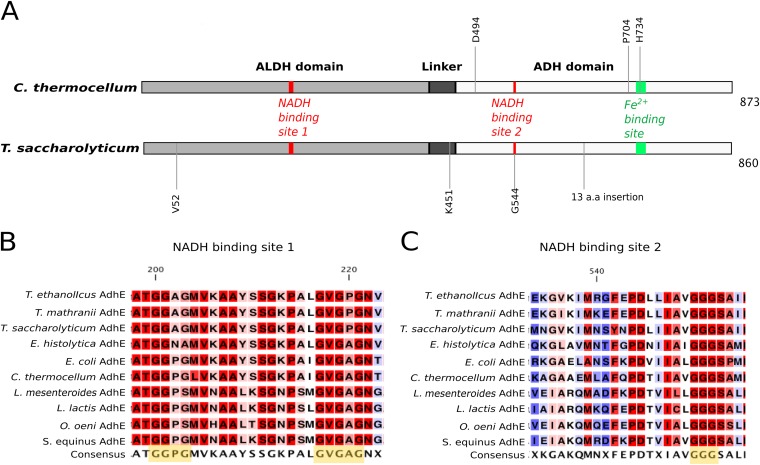
FIG 1.
Primary structures of AdhE proteins of wild-type C. thermocellum and T. saccharolyticum. (A) The ALDH domain is at positions 1 to 423 for C. thermocellum and 1 to 420 for T. saccharolyticum, the ADH domain is at positions 463 to 873 for C. thermocellum and 460 to 860 for T. saccharolyticum, and the linker sequence is at positions 424 to 462 for C. thermocellum and 421 to 459 for T. saccharolyticum. NADH binding site 1 is at positions 200 to 221 for C. thermocellum and 199 to 220 for T. saccharolyticum; NADH binding site 2 is at positions 551 to 553 for C. thermocellum and 543 to 545 for T. saccharolyticum. Mutated residues discussed in this study are annotated at the appropriate positions as follows: D494G in LL350; P704L and H734R in LL346; V52A, K451N, and a 13-amino-acid (a.a.) insertion in LL1040; and G544D in LL1049. All elements are drawn to scale. Panels B and C show the sequence conservation of the NADH binding motifs (highlighted in yellow in the consensus sequence) of AdhE from Thermoanaerobacter ethanolicus, Thermoanaerobacter mathranii, T. saccharolyticum, Entamoeba histolytica, E. coli, C. thermocellum, Leuconostoc mesenteroides, Lactococcus lactis, Oenococcus oeni, and Streptococcus equinus. The residues highlighted in red are the most conserved, and those highlighted in blue are the least conserved. The numbering of amino acids is based on the AdhE sequence of C. thermocellum.