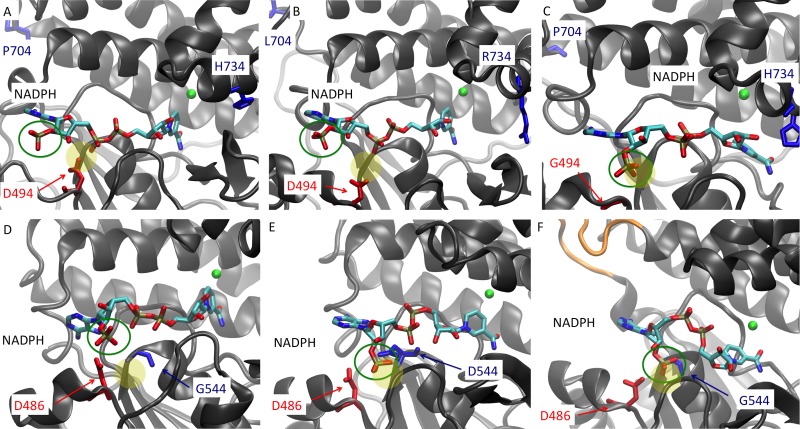
FIG 3.
Average structure of the ADH domains of AdhE from C. thermocellum wild-type LL1004 (A), the ethanol-tolerant LL346 strain (B), moderate ethanol producer LL350 (C), T. saccharolyticum wild-type LL1025 (D), high ethanol producer LL1049 (E), and high ethanol producer LL1040 (F). The amino acids of interest are shown in blue and red; the NADPH cofactor is shown color coded by elements, with its 2′-phosphate group highlighted (green open circle); and the iron ions (green) are also shown. Additionally, the 39-bp insertion in the high ethanol producer LL1040 is shown in orange (F). The yellow-filled circle represents the binding pocket where the additional 2′-phosphate of NADPH is commonly found in NADPH-dependent AdhE proteins. The locations of D494 and D486 are usually the recognition sites for NADH and do not allow the 2′-phosphate group of NADPH (green circle) to access the preferred binding pocket (yellow circle). When the green open circle and the yellow filled circle overlap, that indicates that the NADPH molecule is able to access its preferred binding pocket. This is present in panels C, E, and F but not in the other panels. Panels C, E, and F correspond to enzymes that can use NADPH as a cofactor. Note that NADPH was used for modeling purposes and does not reflect the actual cofactor specificity of the enzymes but rather was used to explain the observed levels of affinity of the enzymes for NADPH.