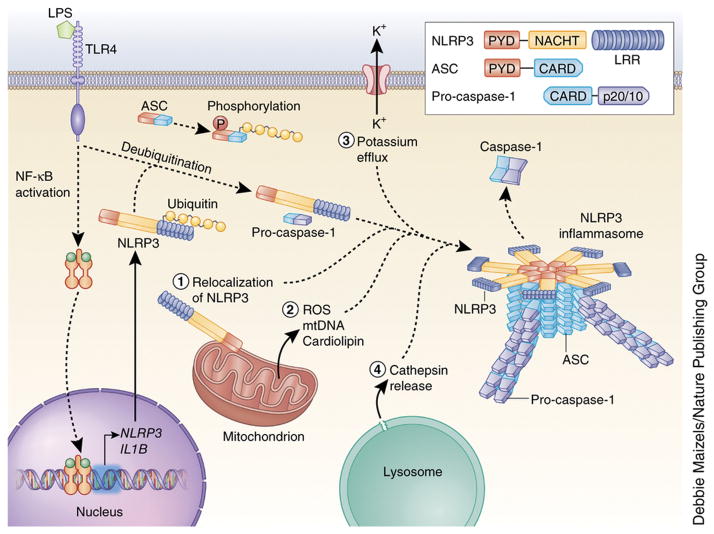
Figure 1. Mechanisms of NLRP3 inflammasome activation.
NLRP3 must be primed before activation. Priming involves two distinct steps. First, an NF-κB–activating stimulus, such as LPS binding to TLR4, induces elevated expression of NLRP3 (as well as IL1B), which leads to increased expression of NLRP3 protein. Additionally, priming immediately licenses NLRP3 by inducing its deubiquitination. The adaptor protein ASC must become linearly ubiquitinated and phosphorylated for inflammasome assembly to occur. After priming, canonical NLRP3 inflammasome activation requires a second, distinct signal to activate NLRP3 and lead to the formation of the NLRP3 inflammasome complex. The most commonly accepted activating stimuli for NLRP3 include relocalization of NLRP3 to the mitochondria, the sensation of mitochondrial factors released into the cytosol (mitochondrial ROS, mitochondrial DNA, or cardiolipin), potassium efflux through ion channels, and cathepsin release following destabilization of lysosomal membranes. Recent studies have determined that activated NLRP3 nucleates ASC into prion-like filaments through PYD-PYD interactions. Pro-caspase-1 filaments subsequently form off of the ASC filaments through CARD-CARD interactions, allowing autoproteolytic activation of pro-caspase-1. Inset shows domain arrangement of the NLRP3 inflammasome components. Pro-caspase-1 and caspase-1 domains are simplified for clarity, the CARD domain is actually removed by cleavage, and two heterodimers form with the p20 and p10 effector domains (p20/10).