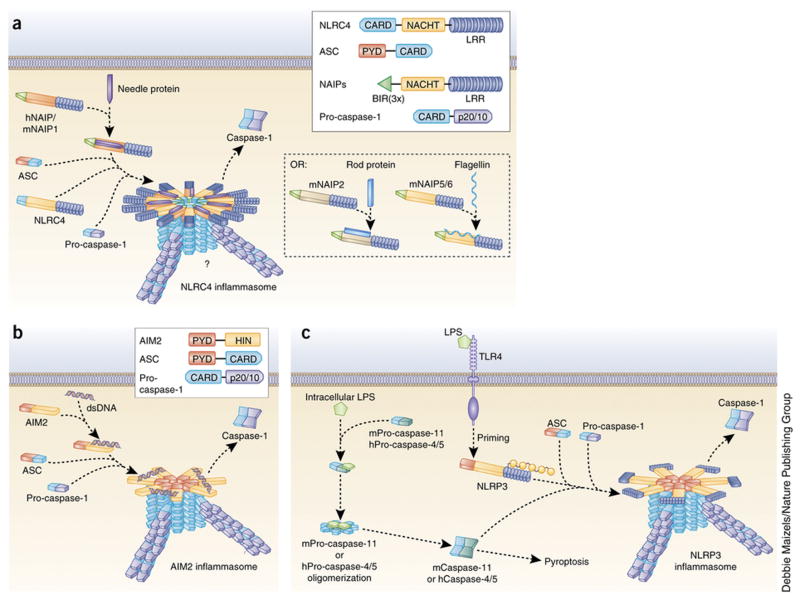
Figure 2. Mechanisms of NLRC4, AIM2 and noncanonical NLRP3 inflammasome activation.
(a) NLRC4 inflammasome agonists such as the bacterial needle protein bind directly to regions within the NACHT domains of the NAIP subfamily of proteins. hNAIP and mNAIP1 bind needle protein, mNAIP2 binds rod protein, and both mNAIP5 and mNAIP6 bind flagellin. Ligand-bound NAIP proteins then oligomerize with NLRC4 to form a caspase-1–activating inflammasome. Though NLRC4 can directly oligomerize with caspase-1 through CARD-CARD interactions, ASC is required for caspase-1 activation by the NLRC4 inflammasome, possibly through the formation of prion-like filaments (blue) by ASC. However, ASC is dispensable for the induction of pyroptosis. Inset shows domain arrangement of NLRC4 inflammasome components. NAIP proteins have three N-terminal BIR domains. hNAIP, human NAIP; mNAIP, mouse NAIP. (b) The mechanism of AIM2 inflammasome activation is well defined. The HIN domain of AIM2 directly binds cytosolic dsDNA, displacing the PYD and relieving autoinhibition. This allows oligomerization of AIM2 PYD with ASC PYD, converting ASC into its prion form. Prion-like filaments of pro-caspase-1 (violet) are then able to form off of the ASC filaments, inducing caspase-1 activation. Inset shows domain arrangement of AIM2 inflammasome components. (c) Studies have determined that mouse pro-caspase-11 (mPro-caspase-11) and human pro-caspases-4 and -5 (hPro-caspase-4/5) can directly bind intracellular LPS and activate a noncanonical NLRP3 inflammasome. This induces oligomerization of these pro-caspases, leading to their proximity-induced activation. This is sufficient for the induction of pyroptosis but not for the processing of pro-IL-1β. However, active mCaspase-11 and hCaspase-4 can promote full assembly and activation of the NLRP3 inflammasome following a priming signal.