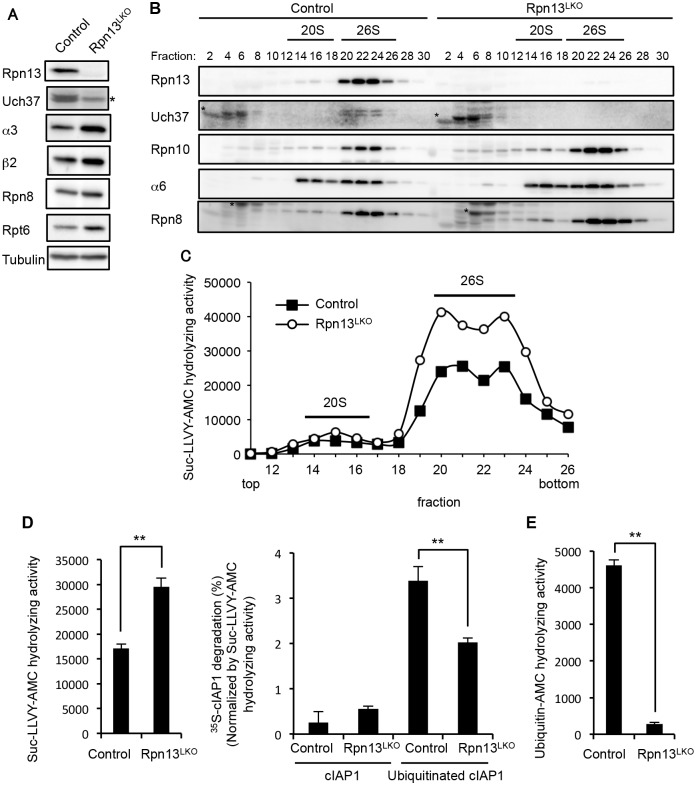
Fig 2. Rpn13 deficiency in the liver impairs degradation of ubiquitinated proteins.
(A) Immunoblot analysis of liver lysates from 8-week-old control and Rpn13LKO mice with antibodies against the indicated proteins. Asterisk indicates a nonspecific band. (B) Lysates from control and Rpn13LKO livers were fractionated by glycerol gradient centrifugation (8 to 32% glycerol from fraction 1 to 30) and an equal amount of each fraction was used for immunoblot analysis using antibodies against the indicated proteins. Asterisks indicate nonspecific bands. (C) Each fraction of (B) was assayed for chymotrypsin-like activity using Suc-LLVY-AMC as a substrate. (D) The 26S proteasome fractions of (C) (fractions 20–23) were subjected to the assay of chymotrypsin-like activity (left panel), and degradation of 35S-labeled cIAP1 with or without ubiquitination was measured and normalized by chymotrypsin-like activity (right panel). Data are mean ±standard deviations from triplicate experiments. **p < 0.01. (E) The deubiquitinating activities of 26S proteasome fractions of (C) were measured using ubiquitin-AMC as a substrate. Data are mean ± standard deviations from triplicate experiments. **p < 0.01