Abstract
Patients with Schizophrenia (SZ) show deficits across various stages of visual information processing. Whether patients with Bipolar Disorder (BD) exhibit these deficits is unclear. In this study, we conducted a detailed comparison of specific stages of early visual perception in BD and SZ. Forty-three BD patients, 43 SZ patients, and 51 matched healthy control subjects (HC) were administered three visual processing paradigms emphasizing: 1) an early stage of object formation (location backward masking), 2) a middle stage of object substitution (four-dot backward masking), and 3) a later stage at the perception-attention interface (rapid serial visual processing (RSVP) task eliciting the attentional blink). SZ performed significantly worse than BD and HC on location and four-dot masking. BD did not significantly differ from HC on either masking task. Both patient groups performed significantly worse than HC on the RSVP task; unlike SZ, BD did not show a significant attentional blink effect compared to HC. Our results indicate that BD patients were intact at the early and middle stages of visual processing (object formation and substitution) but intermediate between the SZ and HC groups at a later processing stage involving perceptual and attentional processes (RSVP task). These findings suggest that SZ is characterized by a diffuse pathophysiology affecting all stages of visual processing whereas in BD disruption is only at the latest stage involving higher order attentional functions.
Keywords: Bipolar disorder, schizophrenia, visual processing, perception, masking, attentional blink
1. Introduction
Bipolar disorder (BD) and schizophrenia (SZ) share many features, including common genetic and psychosocial risk factors (Alloy et al., 2005; Lichtenstein et al., 2009). Impairment in cognition and functional outcome is evident in both disorders, although these deficits are milder and less widespread in BD than in SZ (Bora et al., 2009; Lee et al., 2013). SZ is characterized by deficits in early visual information processing (Butler et al., 2001; Rund et al., 2004). These deficits are associated with impaired higher-level cognition, negative symptoms, and poor functional outcome (Brittain et al., 2010; Green et al., 2012; Rassovsky et al., 2011), and are also linked to specific neural processes (Green et al., 2011a). While visual processing deficits are well-documented in SZ, few studies have examined visual processing integrity in BD.
Visual perception in psychopathology has been frequently assessed with two types of paradigms: visual masking and rapid serial visual processing (RSVP). Visual masking affects two different stages of processing defined by the timing of the mask’s effects on a target: an early object formation stage and a later object substitution stage (Enns, 2004). Masking at the object formation stage occurs roughly between 0–100 ms when the target and mask fuse together to create an integrated composite that is hard to identify. A later stage of processing (100–200 ms) has been assessed via object substitution masking, in which the target percept is replaced by the mask before it reaches awareness. Object substitution masking is thought to interrupt reentrant processing between lower and higher neural levels that are necessary to refine a percept (Enns & Di Lollo, 2000). Four-dot masking is thought to work exclusively through object substitution (Enns & Di Lollo, 1997). Finally, a later stage of visual processing involves interactions between perception and attention and is assessed with RSVP paradigms. During this stage (approximately 200–500 ms) a working memory representation of the first target is established and there is temporary inattention or “attentional blink” (AB) for processing of a second target that appears later (Bachmann & Hommuk, 2005). While these paradigms are designed to emphasize a particular stage of processing they are not always able to rule out the influence of other perceptual or attentional stages of processing.
Visual processing abnormalities have been found in BD. For example, one study (MacQueen et al., 2001) reported impaired object identification and visual location masking in euthymic outpatients with BD. Another (Duffy et al., 2009) found that euthymic BD outpatients with a history of psychosis made more errors than those without a prior history of psychosis on masking. Note that MacQueen et al.’s study only included BD I patients who were not taking antipsychotic medications and that both studies used letters as target stimuli in their masking paradigms. These reports suggest that individuals with BD have deficits in visual perception, but they did not make comparisons with SZ.
Few studies have directly compared visual processing in BD and SZ, and the findings have been mixed. Some studies with hospitalized inpatients reported masking impairment in BD during and soon following a manic episode (Fleming & Green, 1995) that was comparable in magnitude to that seen in SZ (Green et al., 1994). Two studies compared stable outpatients with BD and SZ and reported that BD patients were indistinguishable from non-psychiatric controls on a location masking task (Goghari & Sponheim, 2008; Sponheim et al., 2013), but SZ patients were impaired. Another study reported that BD outpatients showed impairment on an object identification task that was comparable to that of SZ patients (Tam et al., 1998). A more recent study (Chkonia et al., 2012) found deficits on a shine-through masking paradigm in both SZ and BD.
Inconsistency in findings of visual impairment in BD may be due to differences in sample characteristics, task parameters, or the specific visual processing stage that is being assessed. Reliable deficits have been found in SZ, extending from early stages of object formation to later stages of object substitution (Green et al., 2011b) to the interface between perception and attention (Mathis et al., 2011). However, the earliest feedforward stage of visual processing (i.e., from retina to V1) appears to be intact (Jahshan et al., 2012). To our knowledge, no study has examined the pattern of performance of BD and SZ patients as one progresses through visual processing stages.
The aim of this study was to conduct a detailed cross-diagnostic comparison of specific stages of visual processing in stable outpatients with BD and SZ, relative to well-matched healthy controls. Three visual processing measures were administered targeting: 1) an early stage of object formation (location backward masking, first 0–100 ms), 2) a middle stage of object substitution (four-dot masking, 100–200 ms), and 3) a later stage at the perception-attention interface (RSVP task, 200–500 ms).
2. Method
2.1. Participants
The sample consisted of 43 patients with BD I or II, 43 patients with SZ (6 schizoaffective), and 51 healthy controls (HC). SZ and HC participants were a subset drawn from a much larger sample, and data from the complete sample were previously published (Green et al., 2012; Green et al., 2011b; Mathis et al., 2011). These subsamples were selected to be matched to the BD sample on age, gender, and parental education. Data from the BD patients have not been published previously. The test parameters were identical for all samples.
SZ patients were recruited from outpatient clinics at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) and board-and-care residences in the community. BD patients were recruited from mood disorder clinics at the University of California, Los Angeles (UCLA) and the VAGLAHS. Diagnosis was based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 1997). Of the patients with BD, 29 were diagnosed with BD I (7 with a history of psychosis) and 14 with BD II. All patients were considered to be clinically stable, with no medication changes in the past six weeks, no inpatient hospitalization in the past three months, and no changes in housing in the past two months. Additionally, BD patients needed to be out of mood episode. Controls were recruited through internet advertisements and screened with the SCID-I and SCID-II (First et al., 1996). Exclusion criteria for controls were: any lifetime psychotic disorder, bipolar disorder, recurrent depression, substance dependence, paranoid, schizotypal, or schizoid personality disorder, or a reported history of psychotic disorder (including schizophrenia but not bipolar disorder) among first-degree relatives.
Additional exclusion criteria for all groups included being younger than 18 or older than 60 years, an IQ below 70 based on review of medical records, meeting diagnostic criteria for substance dependence in the past 6 months or abuse in the past month, having an identifiable neurological disorder, seizures, history of head injury or loss of consciousness for more than one hour, having less than 20/40 vision as assessed using the Snellen eye chart, or being insufficiently fluent in English, determined by the participant’s ability to understand the consent form. All participants gave written informed consent after receiving a detailed explanation of study procedures in accordance with procedures approved by the Institutional Review Boards at UCLA and VAGLAHS.
For SZ patients, 37 were receiving second-generation antipsychotic medications, 1 a first-generation and 3 both types at the time of testing. One was not taking medication and 1 was missing medication information. For BD patients, most were receiving more than one psychoactive drug: 27 were taking antipsychotic medications, 21 were taking anticonvulsants, 12 were taking lithium, and 17 were taking antidepressants. Two were not taking medications and 1 was missing medication information.
2.2. Assessments
2.2.1. Clinical ratings
SZ received the 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993b) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984). We report the positive symptoms and depression/anxiety factors, as well as the total score for the BPRS (Kopelowicz et al., 2008) in Table 1. BD received the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) and the Young Mania Rating Scale (YMRS; Young et al., 1978). Clinical assessments were conducted by interviewers trained to reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center based on established procedures (Ventura et al., 1993a; 1998).
Table 1.
Demographic and Clinical Characteristics
| Healthy Controls (N=51) |
Patients with BD (N=43) |
Patients with SZ (N=43) |
|
|---|---|---|---|
| Age (Mean/SD) | 42.0 (9.1) | 42.7 (10.7) | 45.6 (7.8) |
| Gender (M:F) | 32:19 | 25:18 | 24:19 |
| Personal Education (Mean/SD)** | 14.5 (1.7) | 14.4 (2.4) | 12.5 (1.8) |
| Parental Education (Mean/SD) | 14.2 (2.7) | 14.9 (3.1) | 14.1 (2.8) |
| Race (% White) | 54.9% | 55.8% | 37.2% |
| BPRS Positive Symptoms (Means/SD)** | --------- | 8.0 (1.4) | 15.6 (6.4) |
| BPRS Depression/Anxiety (Mean/SD) |
--------- | 8.3 (3.7) | 8.1 (3.4) |
| Total BPRS (Mean/SD)** | --------- | 33.2 (7.3) | 44.7 (10.5) |
| Total SANS (Mean/SD) | --------- | --------- | 7.4 (3.5) |
| Total HDRS (Mean/SD) | --------- | 7.3 (6.8) | --------- |
| Total YMRS (Mean/SD) | --------- | 3.1 (4.2) | --------- |
BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms; HDRS = Hamilton Depression Rating Scale; YMRS = Young Mania Rating Scale.
p < 0.001
2.2.2. Visual perceptual measures
For all three tasks, participants sat 1 m from a 17 inch cathode ray tube monitor with a refresh rate of 160 Hz (6.25 ms per screen sweep). Stimuli were presented using E-Prime 1.1 (Psychological Software Tools, Pittsburgh, PA). Participants provided verbal responses to the experimenter who then recorded those responses.
2.2.2.1. Location backward masking
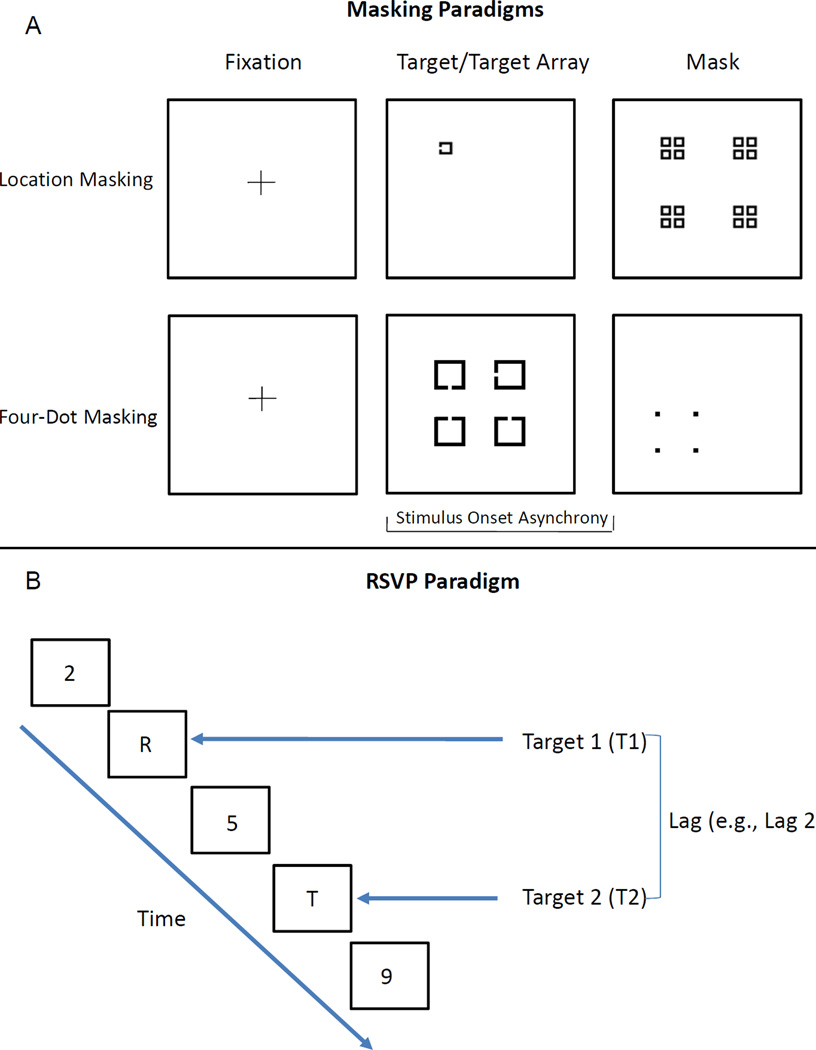
This procedure has been described in detail elsewhere (Green, et al., 2011b). In this task the target consisted of a square with a notch that could appear at the top, bottom, or left side of the square. The target could appear at one of four different locations, arranged in a notional square, on the screen. The masking stimulus consisted of a pattern of squares that occupied every possible target location. Targets measured 0.27 × 0.27° of visual angle and the mask measured 2.01 × 2.01°. Examples of the stimuli are shown in Figure 1A. The target was presented for 12.5 ms and the mask for 25 ms. Backward masking (mask following target) was assessed using 7 stimulus onset asynchronies (SOAs) ranging from 0 (simultaneous target and mask onset) to 75 ms. Twelve trials were presented for each SOA. Performance is lowest at the SOA of 0 ms and increases with increasing SOAs. Each trial started with a 400 ms fixation cross followed by a 500 ms blank screen. Participants reported in which one of the four quadrants the target appeared. Prior to the masking task, target contrast was set to each subject’s threshold so that all subjects identify the unmasked target with 84% accuracy. The thresholding procedure was a three up one down staircase procedure where the contrast of the target was increased or decreased.
Figure 1.
Examples of the stimuli used in the masking (A) and RSVP (B) paradigms.
2.2.2.2. Four-dot masking
This procedure has been described in detail elsewhere (Green, et al., 2011b). In this task, four potential targets appeared in a notional square followed by a mask that surrounds one potential target and cues which target is to be identified. The targets were four squares with a notch missing from one side. The mask consisted of four dots that surround, but do not touch, one of the potential targets. Each potential target measured 1.55 × 1.55° of visual angle and was arran ged in a square of 4.58 × 4.58°. The four-dot mask measured 2.23 × 2.23° of visual a ngle and each dot in the mask subtended 0.23 × 0.23°. Examples of the stimuli are shown in Figure 1A. The target was presented for 25 ms and the mask was presented for 37.5 ms. The target and masking stimuli were separated by eight SOAs ranging from 0 to 175 ms. Performance typically decreases with increasing SOAs, unlike location masking. Twelve trials were presented for each SOA. Each trial started with a 450 ms fixation cross ms followed by a 500 ms blank screen. Participants reported the direction of the notch (up, side, or down) of the target that was surrounded by the four-dot mask.
2.2.2.3. RSVP task
This procedure has been described in detail elsewhere (Mathis, et al., 2011). This paradigm consists of a single target task that measures basic visual perception and a dual target task that elicits the attentional blink (AB) effect. The AB typically occurs when the interval between T1 and T2 is roughly 200–500 ms, resulting in decrease in accuracy for identifying T2 (Dux & Marois, 2009). For both tasks, each trial began with a 500 ms fixation cross followed by a 400 ms blank screen. Each stimulus subtended 2° of visual angle and was displayed for 62.5 ms with an inter-stimulus interval of 25 ms. In the single target task, a target (letter) was presented among a rapid stream of distracting stimuli (numbers) and participants were asked to identify the letter. In the dual target task, two targets (T1 and T2) were presented either with no intervening distractors (lag 1) or separated by 1 (lag 2), 2, 3, 4, 5, 7, 9, or 11 (lag 12) distractors. Ten trials were administered in the single target task and ten trials for each lag in the dual target task. Examples of the stimuli are shown in Figure 1B. In both tasks after each trial a screen displaying all of the possible targets appeared prompting the subjects to make their response. Participants named both target letters in the order that they were presented.
2.3. Data analysis
One-way analyses of variance (ANOVAs) and chi-square tests were used to assess group differences for continuous and categorical demographic variables, respectively. A one-way ANOVA was conducted for unmasked location performance. We performed a 3 (group) × 7 (SOA) ANOVA for backward masking and a 3 (group) × 8 (SOA) ANOVA for four-dot masking. For the RSVP task, we conducted a one-way ANOVA for the single target task, and a 3 (group) × 9 (lag) ANOVA for the dual target task. The dependent variable was the conditional probability for correctly identifying the second target given the correct identification of the first target: P (T2/T1) (Chun & Potter, 1995).
We also calculated the suppression ratio (SR) (cf. Cheung et al., 2002) at each lag to assess the AB effect after controlling for group differences on the single target RSVP task. The SR is the degree of performance change in the dual target task relative to the single target task, with scores ranging from 0 to 1. The SR was calculated as follows: SR at lag X = P (T2/T1) at lag X divided by [P (T1) on single target task + P (T2/T1) at lag X], and analyzed with a 3 (group) × 9 (lag) ANOVA.
Least significant difference (LSD) tests were conducted as post hoc analyses to follow up on significant main effects. We performed independent samples t-tests within the BD group to examine performance differences between 1) BD I versus BD II, 2) BD I with versus without history of psychosis, 3) those taking versus not taking lithium, and 4) those taking versus not taking antipsychotic medications. Lastly, we conducted Pearson’s correlations between each task and symptom ratings within each patient group.
3. Results
3.1. Demographic and clinical characteristics
Demographic and symptom ratings can be seen in Table 1. The groups were matched on age, gender distribution, race, and parental education. The groups differed on personal education (F [2, 134] = 14.32, p < 0.001) with SZ having significantly fewer years of education than both BD and HC (p’s < 0.001). SZ had more severe overall symptoms (BPRS: t [82] = 5.78, p < 0.001) than BD. BD had minimal to mild levels of depressive and manic symptoms. We examined gender as a factor but found no significant main effects of gender or group X gender interactions in any of the ANOVAs and decided to exclude it from further analyses.
3.2. Visual processing
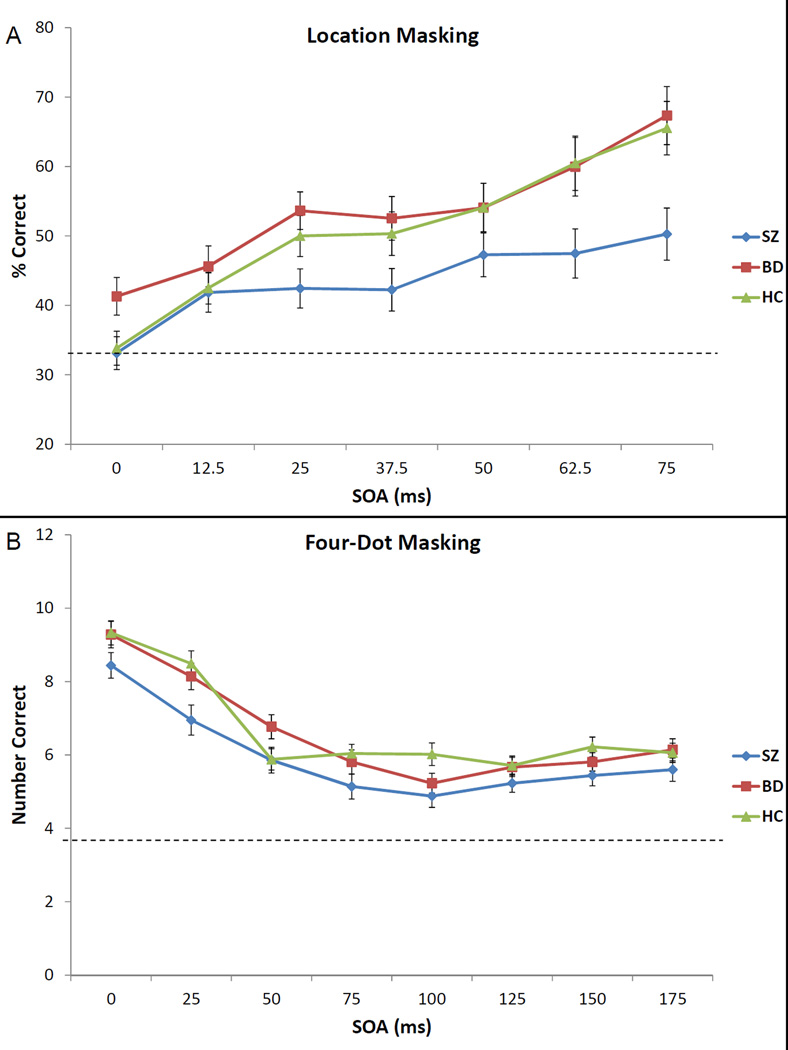
3.2.1. Location backward masking
Results are shown in Figure 2A and Table 2. The ANOVA revealed significant main effects of group (F [2, 134] = 4.79, p = 0.01) and SOA (F [6, 804] = 32.65, p < 0.001) but no significant group X SOA interaction (F [12, 804] = 1.53, p = 0.11). The main effect of group was because SZ performed significantly worse than HC (p = 0.02, d = 0.49) and BD (p = 0.004, d = 0.67). BD did not significantly differ from HC (p = 0.43, d = − 0.15).
Figure 2.
Performance of the bipolar group (in red), schizophrenia group (in blue), and healthy control group (in green) on location backward masking (A) and four-dot masking (B). The bars represent standard errors and the dotted line represents chance performance.
Table 2.
Means and Standard Deviations for Visual Processing Measures
| Healthy Controls (N=51) |
Patients with bipolar disorder (N=43) |
Patients with schizophrenia (N=43) |
|
|---|---|---|---|
| Location Masking (% accuracy) | |||
| Backward Masking | 50.96 (16.67) | 53.50 (16.01) | 43.53 (13.74)a,c |
| Unmasked Performance | 96.57 (8.02) | 95.00 (10.15) | 91.28 (11.92)a |
| Four-Dot Masking (# correct) | 6.72 (1.45) | 6.61 (1.29) | 5.94 (1.43)a,c |
| Attentional Blink | |||
| Single Target Task | 0.85 (0.09) | 0.78 (0.15)a | 0.73 (0.15)b,c |
| Dual Target Task (lags 2–5) | 0.59 (0.21) | 0.51 (0.21)a | 0.40 (0.19)b,c |
| Suppression Ratio (lags 2–5) | 0.39 (0.09) | 0.36 (0.11) | 0.32 (0.11)b |
significantly different from HC group (p < 0.05)
significantly different from HC group (p < 0.001)
significantly different from BD group (p < 0.05)
Although all groups’ unmasked performance was near ceiling, there was a significant difference on this measure (F [2, 134] = 3.35, p = 0.04) with SZ performing significantly worse than HC (p = 0.01), but not BD (p = 0.09). There was no significant difference between BD and HC (p = 0.45).
3.2.2. Four-dot masking
All groups showed the expected decrease in performance with increasing SOAs over the first four SOAs (Figure 2B). The ANOVA revealed significant main effects of group (F [2, 134] = 4.04, p = 0.02) and SOA (F [7, 938] = 82.75, p < 0.001) but no significant group X SOA interaction (F [14, 938] = 1.50, p = 0.10). Pairwise comparisons showed that SZ performed significantly worse than HC (p = 0.01, d = 0.54) and BD (p = 0.03, d = 0.49). The BD and HC groups did not differ (p = 0.70, d = 0.08). Means and standard deviations for the average across SOAs are reported in Table 2.
3.2.3. RSVP task
The ANOVA for the single target task revealed a significant main effect of group (F [2, 134] = 10.49, p < 0.001) with SZ identifying fewer targets than both HC (p < 0.001, d = 0.97) and BD (p = 0.05, d = 0.33). BD also identified fewer targets than HC (p = 0.01, d = 0.57) (see Table 2).
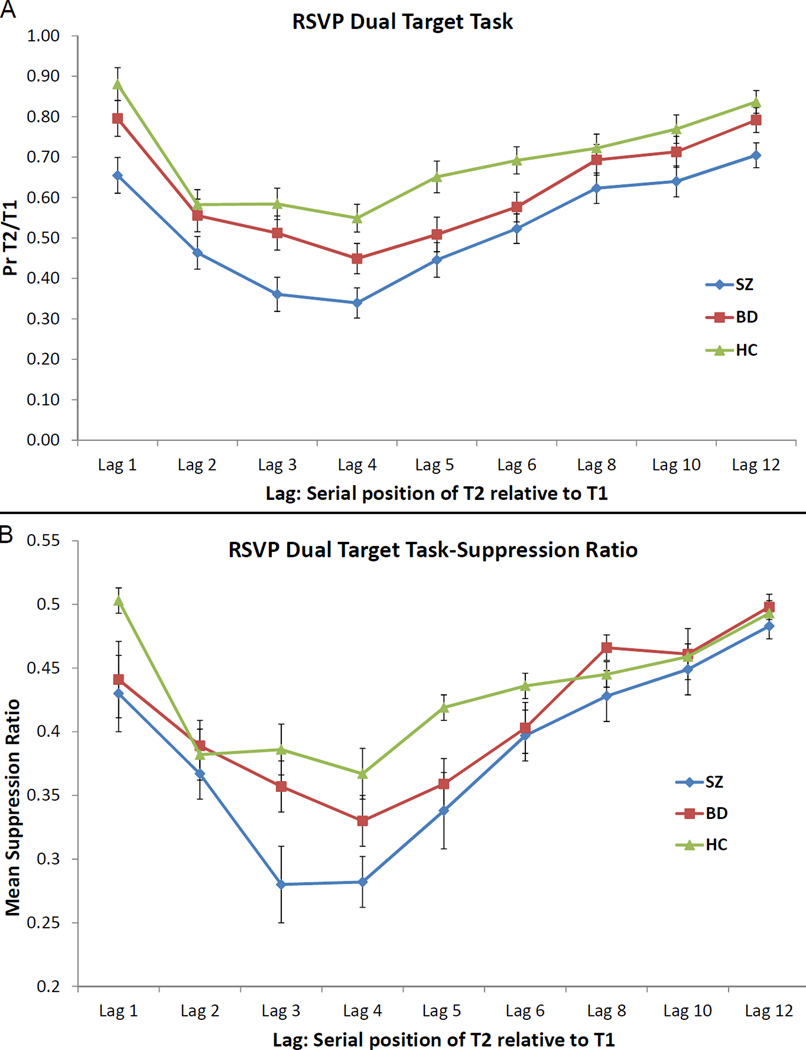
The ANOVA for the dual target task revealed significant main effects of group (F [2, 134] = 10.96, p < 0.001) and lag (F [8, 1072] = 52.51, p < 0.001) but no group X lag interaction (F [16, 1072] = 1.00, p = 0.46). Looking at the mean of lags 2 to 5 where the AB effect occurs, we found a significant effect of group (F [2, 134] = 9.76, p < 0.001). This effect was because SZ performed significantly worse than HC (p < 0.001, d = 0.95) and BD (p = 0.02, d = 0.55). BD’s performance was significantly worse than that of HC (p = 0.049, d = 0.38). All groups showed a clear decline in performance from lag 1 to lag 2, with a minimum attained at lag 4 (Figure 3A).
Figure 3.
Performance of the bipolar group (in red), schizophrenia group (in blue), and healthy control group (in green) on the RSVP dual target task, using the conditional probability of correct T2 identification given correct T1 identification (A) and the suppression ratio (B). The bars represent standard errors.
The ANOVA for the SR revealed significant main effects of group (F [2, 134] = 4.57, p = 0.01) and lag (F [8, 1072] = 36.25, p < 0.001) and a significant group X lag interaction (F [16, 1072] = 1.81, p = 0.02) (Figure 3B). Looking at the mean of lags 2 to 5, we found a significant effect of group (F [2, 134] = 5.63, p = 0.004). This effect was due to SZ performing significantly worse than HC (p = 0.001, d = 0.70); BD showed a trend level difference with SZ (p = 0.06, d = 0.36). BD and HC did not differ from each other (p = 0.17, d = 0.30).
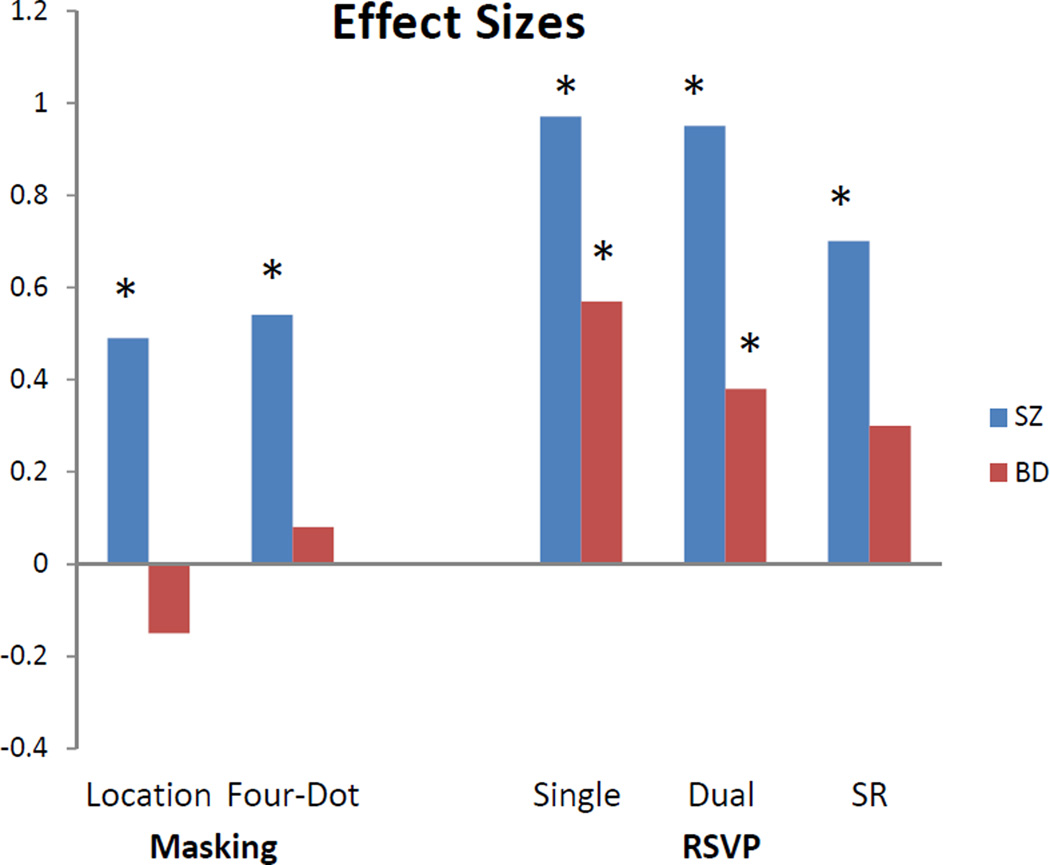
3.3. Effect sizes
To examine the magnitude of difference in performance between the patient groups and controls as one progresses through visual processing stages, we computed effect sizes for each task. The SZ group showed medium effect sizes on the masking tasks and large effect sizes on the RSVP tasks (Figure 4). The BD group followed a different pattern, namely small effect sizes on the masking tasks, a medium effect size on the RSVP single target task, and a small-medium effect size on the dual target task.
Figure 4.
Cohen’s d effect sizes for the two patient groups relative to the healthy control group on each of the visual processing tasks. These effect sizes are based on the mean of the seven SOAs for location masking, the mean of the eight SOAs for four-dot masking, the mean score on the RSVP single target task, and the mean of lags 2 to 5 for the dual target task and SR. The asterisks represent which effect sizes issued forth significant differences.
3.4. Subgroup analyses with BD
We found no significant differences on any of the visual processing measures between BD I versus BD II patients (all p’s > 0.20), BD I patients with versus without history of psychosis (all p’s > 0.25), BD patients taking versus not taking lithium (all p’s > 0.12) and BD patients taking versus not taking antipsychotic medications (all p’s > 0.19).
3.5. Correlations with clinical symptoms
We found no significant associations between the visual processing measures and BPRS in both groups, SANS in the SZ group or HDRS/YMRS in the BD group.
4. Discussion
This is the first study to directly compare performance of patients with BD and SZ on specific stages of visual processing. The pattern of performance for the two patient groups differed as they progressed through visual processing stages. The effect sizes between the SZ and control groups were medium to large across tasks. The BD group did not significantly differ from the control group on the masking tasks but showed medium and small to medium effect-size deficits on the RSVP single target task and dual target task, respectively. Both patient groups showed larger effect sizes on the task with the latest stage of processing (RSVP), especially on the single target task. These results suggest that deficits in SZ are apparent at early and later stages of visual processing, whereas processing is intact in BD with deficits only appearing at later stages. When subgroups of BD were considered, there were no significant differences on any of the visual processing measures between patients with BD I versus BD II disorder, BD I with versus without history of psychosis, those taking versus not taking lithium, or those taking versus not taking antipsychotic medications.
The results in BD patients are consistent with two other studies of visual processing showing intact early processing using a location masking task (Goghari & Sponheim, 2008; Sponheim et al., 2013); however, our results are inconsistent with those from two different studies that found impaired processing in BD using an object identification task (Tam et al., 1998) and a shine-through masking paradigm (Chkonia et al., 2012). The paradigms in those two studies differed from our masking tasks in several aspects (e.g., only 2 SOAs, pairs of digits as targets, discrimination threshold procedure). Moreover, Chkonia et al.’s BD sample was more symptomatic and included inpatients. Our findings extend the previous findings by showing that BD patients are comparable to controls on early and middle stages (i.e., object formation and object substitution, respectively).
In terms of the RSVP task (not included in the previous studies), the BD group significantly differed from the other two groups on the single and dual target tasks. BD patients’ performance did not seem to worsen with more complex task demands as seen by a smaller effect size for the dual target task versus the single target task. Although BD patients performed poorly on the RSVP tasks, the lack of a significant difference in the suppression ratio between the BD and control groups suggests that the more pronounced attentional blink effect in BD is largely due to deficits in the single target task. Therefore, the BD group’s reduced accuracy at detecting the second target is not related to the attentional blink itself but rather to a general deficit in sustaining attention or maintaining the target in a working memory stage that occurs after object formation.
BD patients’ deficient target identification in the single target task in the context of intact masking could also be explained by differences between the masking and RSVP paradigms. These measures clearly involve different demands as the SZ patients’ performance worsened (the effect size almost doubled) on the RSVP compared to the masking tasks. Masking may require more spatial information (e.g., locate a notch), whereas RSVP may require form discrimination. Moreover, RSVP tasks require sustained orientation or alertness to visual stimuli, whereas masking involves a single stimulus array preceded by a fixation point and followed by a mask.
Although SZ patients showed poor performance across all stages of visual processing, there is evidence that very early feedforward processing before the formation of percepts is intact in SZ (Green et al., 2009; Hahn et al., 2011; Jahshan et al., 2012). Taking into account these previous studies and the current study, it is likely that SZ patients are impaired at all subsequent stages beyond the initial sensory feedforward processing stage.
Our study is limited by the fact that SZ patients were taking antipsychotic medications at the time of testing. However, given that antipsychotic medications did not have an effect on visual processing in BD, and previous studies of visual masking in SZ found no effects of antipsychotic medications (Butler et al., 1996; Green et al., 1999), it is unlikely that these medications had a strong impact on the findings. Although lithium may have an effect on visual processing (Fleming & Green, 1995; Green, et al., 2011b), only 28% of our BD patients were taking lithium and their performance was not significantly different than those not taking lithium.
In summary, the BD group’s performance was comparable to the control group at the earliest stages of perceptual processing (object formation and substitution) but intermediate between the SZ and control groups at a later processing stage involving the interface between perception and higher order processing. These results suggest that, in contrast to the widespread disruption of neural processes across early and later stages of the visual processing hierarchy observed in SZ, neural processes associated with early stage visual processing are intact in BD. However, reduced performance on the RSVP task is consistent with narrow disruption of higher order attentional networks in BD. Despite the commonly-reported neurocognitive and genomic similarities between SZ and BD (Bora et al., 2009; Purcell et al., 2009), deficient early visual processing does not seem to be a prominent characteristic in BD patients and does not represent a shared phenotype between the two patient groups. Given models of pathways from early visual perception to functional outcome in SZ (Green et al., 2012), future studies might benefit from evaluating cascade models in BD starting with the latest stage of visual information processing.
Acknowledgements
This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment. The authors would like to thank Crystal Gibson, Cory Tripp, Katie Weiner, Mark McGee, Christen Waldon and Amanda Bender for their assistance with recruitment and testing.
Role of Funding Source
Funding for this study was provided by NIH Grants MH089634 and MH43292 (MFG). The NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MFG and JKW designed the study. CJ and JKW analyzed the data. CJ, JKW, AM, and MFG interpreted the findings and wrote the first draft of the manuscript with DCG and LLA providing feedback. All authors have contributed to and approved the final manuscript.
References
- Alloy LB, Abramson LY, Urosevic S, Walshaw PD, Nusslock R, Neeren AM. The psychosocial context of bipolar disorder: environmental, cognitive, and developmental risk factors. Clinical Psychology Review. 2005;25:1043–1075. doi: 10.1016/j.cpr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Bachmann T, Hommuk K. How backward masking becomes attentional blink. Perception of successive in-stream targets. Psychological Science. 2005;16:740–742. doi: 10.1111/j.1467-9280.2005.01604.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. British Journal of Psychiatry. 2009;195:475–482. doi: 10.1192/bjp.bp.108.055731. [DOI] [PubMed] [Google Scholar]
- Brittain P, Ffytche DH, McKendrick A, Surguladze S. Visual processing, social cognition and functional outcome in schizophrenia. Psychiatry Research. 2010;178:270–275. doi: 10.1016/j.psychres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Butler PB, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: Relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biological Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Cheung V, Chen EY, Chen RY, Woo MF, Yee BK. A comparison between schizophrenia patients and healthy controls on the expression of attentional blink in a rapid serial visual presentation (RSVP) paradigm. Schizophrenia Bulletin. 2002;28:443–458. doi: 10.1093/oxfordjournals.schbul.a006952. [DOI] [PubMed] [Google Scholar]
- Chkonia E, Roinishvili M, Reichard L, Wurch W, Puhlmann H, Grimsen C, Herzog MH, Brand A. Patients with functional psychoses show similar visual backward masking deficits. Psychiatry Research. 2012;198:235–240. doi: 10.1016/j.psychres.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology. Human Perception and Performance. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Duffy A, Hajek T, Alda M, Grof P, Milin R, MacQueen G. Neurocognitive functioning in the early stages of bipolar disorder: visual backward masking performance in high risk subjects. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:263–269. doi: 10.1007/s00406-008-0862-3. [DOI] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: a review of data and theory. Attention, Perception & Psychophysics. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns JT. Object substitution and its relation to other forms of visual masking. Vision Research. 2004;44:1321–1331. doi: 10.1016/j.visres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V. Object substitution: A new form of masking in unattended visual locations. Psychological Science. 1997;8:135–139. [Google Scholar]
- Enns JT, Di Lollo V. What's new in visual masking? Trends in Cognitive Science. 2000;4:345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York: State Psychiatric Institute; 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York: State Psychiatric Institute; 1997. [Google Scholar]
- Fleming K, Green MF. Backward masking performance during and after manic episodes. Journal of Abnormal Psycholology. 1995;104:63–68. doi: 10.1037//0021-843x.104.1.63. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Sponheim SR. Divergent backward masking performance in schizophrenia and bipolar disorder: association with COMT. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147:223–227. doi: 10.1002/ajmg.b.30583. [DOI] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Archives of General Psychiatry. 2012;69:1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC. Functional neuroanatomy of visual masking deficits in schizophrenia. Archives of General Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: Overview and theoretical implications. Schizophrenia Bulletin. 2011a;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: Possible reflections of aberrant cortical oscillations. American Journal of Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: Specifying a mechanism. Archives of General Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Green MF, Wynn JK, Breitmeyer B, Mathis KI, Nuechterlein KH. Visual masking by object substitution in schizophrenia. Psychological Medicine. 2011b;41:1489–1496. doi: 10.1017/S003329171000214X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Kappenman ES, Robinson BM, Fuller RL, Luck SJ, Gold JM. Iconic decay in schizophrenia. Schizophrenia Bulletin. 2011;37:950–957. doi: 10.1093/schbul/sbp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Breitmeyer BG, Green MF. Nonconscious and conscious color priming in schizophrenia. Journal of Psychiatric Research. 2012;46:1312–1317. doi: 10.1016/j.jpsychires.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. American Journal of Psychiatry. 2013;170:334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Young LT, Galway TM, Joffe RT. Backward masking task performance in stable, euthymic out-patients with bipolar disorder. Psychological Medicine. 2001;31:1269–1277. doi: 10.1017/s0033291701004597. [DOI] [PubMed] [Google Scholar]
- Mathis KI, Wynn JK, Breitmeyer B, Nuechterlein KH, Green MF. The attentional blink in schizophrenia: isolating the perception/attention interface. Journal of Psychiatric Research. 2011;45:1346–1351. doi: 10.1016/j.jpsychires.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychological Medicine. 2011;41:487–497. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund BR, Egeland J, Sundet K, Asbjornsen A, Hugdahl K, Landro NI, Lund A, Roness A, Stordal KI. Early visual information processing in schizophrenia compared to recurrent depression. Schizophrenia Research. 2004;68:111–118. doi: 10.1016/S0920-9964(03)00193-2. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Sass SM, Noukki AL, Hegeman BM. Fragile early visual percepts mark genetic liability specific to schizophrenia. Schizophrenia Bulletin. 2013;39:839–847. doi: 10.1093/schbul/sbs041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WC, Sewell KW, Deng HC. Information processing in schizophrenia and bipolar disorder: a discriminant analysis. Journal of Nervous and Mental Disease. 1998;186:597–603. doi: 10.1097/00005053-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: 'the drift busters'. International Journal of Methods in Psychiatric Research. 1993a;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993b;3:227–243. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]