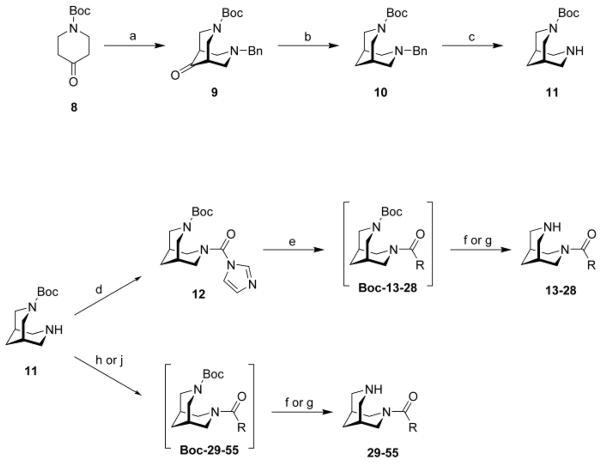
Scheme 2.
Synthesis of bispidine derivatives. Reagents and conditions: (a) (CH2O)n, BnNH2, AcOH, MeOH, [Ar] reflux, 6 h; (b) N2H4 (80%), NaOH, diethylene glycol, 125 °C, 2 h, then Dean Stark trap, 140 °C, 8 h; (c) Pd/C (5%), H2 (2–4 bar), MeOH, rt, 4–24 h; (d) CDI, THF, reflux, 2 h; (e) (i) MeI, MeCN, THF, rt, 24 h; (ii) R-COOH, Et3N, MeCN, rt, 12–120 h; (f) HCl/1,4-dioxane (4M), rt, 4 h; (g) anhydr. ZnBr2, CH2Cl2, rt, 12–120 h; (h) R-COCl, Et3N, toluene, rt, 2h; (j) R-COOH, DCC, DMAP, CH2Cl2, 0 °C to rt, 12 h.