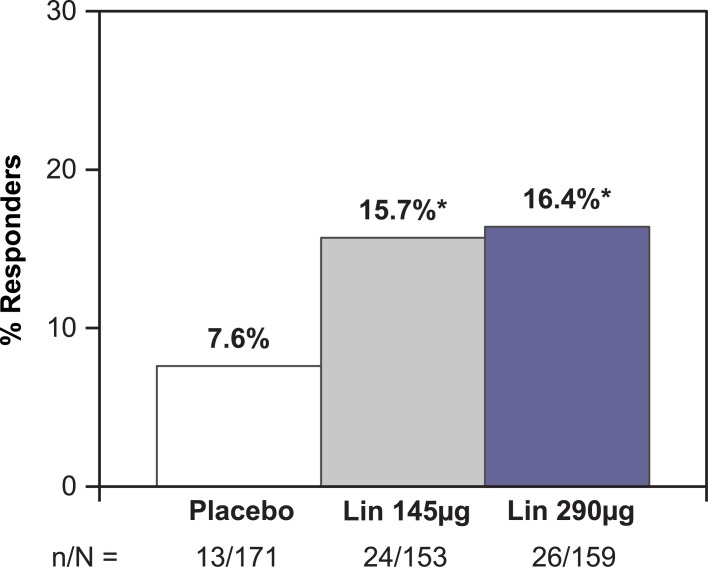
Fig 2. Primary Endpoint.
Intent-to-treat Population; Responder = patient who had ≥ 3 CSBMs and an increase of ≥ 1 CSBM from baseline, in the same week, for at least 9 of the 12 treatment-period weeks. Note: Primary endpoint for linaclotide 145 μg vs. placebo; secondary endpoint for linaclotide 290 μg vs. placebo. CSBM = complete spontaneous bowel movement; ITT = intent to treat; Lin = linaclotide; n = number of patients meeting the responder endpoint; N = number of patients in the ITT population. * P < 0.05; P values were obtained from a Cochran-Mantel-Haenszel test controlling for geographic region, comparing each linaclotide dose vs. placebo in a pairwise manner.