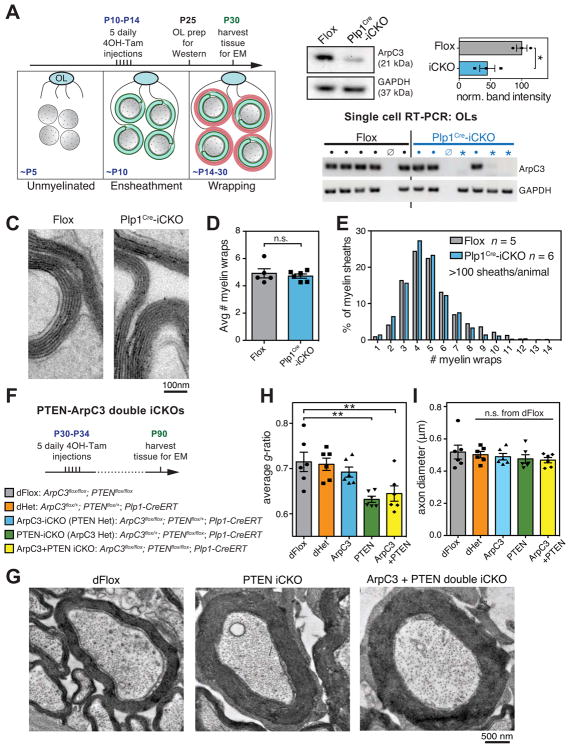
Figure 4. Arp2/3 is dispensable for myelin wrapping.
(A) Experimental paradigm for tamoxifen injections to ablate ArpC3 during myelin wrapping.
(B) Reduction of ArpC3 mRNA and protein in OLs purified at P25 from ArpC3Flox/Flox (Flox) and ArpC3Flox/Flox; Plp1-CreERT (induced CKO, Plp1Cre-iCKO) mice that were injected with tamoxifen from P10–14. Top left, immunoblotting of ArpC3 from immunopanned OLs. Top right, densitometry of ArpC3 protein in OLs purified from n = 3 animals per genotype; *p < 0.05, Student’s t-test. Bottom, single cell RT-PCR analysis shows ArpC3 knockout OLs (GAPDH but no ArpC3, *), WT OLs (both GAPDH and ArpC3 bands, ●), or no cells (neither GAPDH nor ArpC3, ø). n > 22 reactions per animal, from one Flox and two Plp1Cre-iCKO mice.
(C–E) Transmission electron microscopy of P30 optic nerves showed no difference in number of myelin wraps in Plp1Cre-iCKOs. (C), example micrographs, (D), average number of myelin wraps per animal, and (E), distribution of number of wraps in all animals. Error bars: SEM from n = 5–6 animals per genotype; >100 myelin sheaths per animal; n.s. = not significant, Student’s t-test.
(F) Experimental paradigm for tamoxifen injections to ablate PTEN and/or ArpC3 at P30, and full genotypes of mice.
(G) Transmission electron microscopy of optic nerves at P90 showing increased myelin wrapping in PTEN-iCKO (middle) and ArpC3+PTEN double iCKO (right) mice, compared to double floxed controls (left).
(H) g-ratio analysis of optic nerves at P90 shows lower g-ratios after deletion of PTEN or both ArpC3+PTEN.
(I) Axon diameter was unaffected.
Error bars: SEM from n = 6 animals per genotype; >100 myelin sheaths measured per animal; n.s. = not significant, *p < 0.05, **p < 0.01, Dunnett’s multiple comparison test.
See also Figure S5.