Abstract
The present study was undertaken with objectives of; a) to investigate and compare Pseudomonas aeruginosa isolates from two dairies for biofilm formation potential and, b) to compares three common biocides for biofilm eradication efficiencies. Amongst the isolates from commercial dairy, 70 % were strong and/or moderate biofilm former in comparison to 40 % isolates from small scale dairy. All isolates, irrespective of source, exhibited higher susceptibility to biocides in planktonic stage than in biofilm. Antibiofilm efficiencies of three biocides i.e. benzalkonium chloride, sodium hypochlorite and iodophore were determined in terms of their microbial biofilms eradicating concentration (MBEC). Our findings show that the three biocides were ineffective against preformed biofilms at recommended in-use concentrations. Biofilms were the most resistant to benzalkonium chloride and least against iodophore. A trend of decreasing MBECs was observed with extended contact time. The findings of present study warrant for a systematic approach for selecting types and concentrations of biocide for application as antibiofilm agent in food industry.
Keywords: Dairy biofilm, P. aeruginosa, Benzalkonium chloride, Iodophore, Sodium hypochlorite, Biocide
Introduction
Recent realization of the roles a food chain plays in spread and emergence of antimicrobial resistance has emphasized need for better understanding of related behaviors of food microflora. Pseudomonas aeruginosa is gram negative, non-spore former, oxidase positive organism which has been a part of natural microflora of vegetables, meat, milk and various food products (Franzetti and Scarpellini 2007). It is considered to be causative of off flavors and unpleasant textural changes in finished food products (Martin et al. 2011; Van Tassell et al. 2012). Although rarely associated with foodborne illnesses, the organism is a leading cause of nosocomial infections and possesses inherent antimicrobial resistance (Toté et al. 2010). Multidrug resistance of P. aeruginosa strains has been associated in general with their potential to form a protective matrix of extracellular material, known as biofilm. Strong biofilm formation potential (BFP) of P. aeruginosa has been reported to be instrumental in surviving harsh conditions such as high processing temperatures, low water activity, low storage temperatures and routine cleaning-in-place (CIP) procedures in food industry (Chmielewski and Frank 2003). Different dairy plants generally differ in milk handling capacity, instrumentation, and product profile and processing facilities. Therefore, microflora of such dairies is expected to vary in number and diversity of representative communities. Such microflora may also differ substantially in their behaviors such as BFP and resistance to antimicrobial compounds. In spite the fact, hardly any study so far has compared microflora of different dairies for BFP. In this direction, the current study attempts to compare P. aeruginosa isolates from four different isolation points of two dairies for BFP.
For biofilm control and eradication, various strategies have been explored in food industry. Incorporation of biocides such as benzalkonium chloride (BC), sodium hypochlorite (SH) and iodophore (ID) etc. in CIP regime is one of the common strategies. Generally, antibiofilm efficiencies of antimicrobial agents are governed by a number of factors (Chmielewski and Frank 2003). The most important one is biofilm matrix, which by acting as a physical barrier decreases penetration of antimicrobial agents (Toté et al. 2010). In addition, various circumstances such as carry over contamination of water, improper rinse, and protective action of foods may also reduce the effective concentration of antimicrobial agents to sub-lethal levels. Exposure of organisms to these sub-lethal antimicrobial concentrations has been reported to adapt them to higher concentrations of related and unrelated compounds and thus giving rise to adaptive and cross resistant microbial population (van der Horst et al. 2011; Pagedar et al. 2011). Such population may help in emergence and spread of antimicrobial resistance through food chain.
Antibiofilm efficiencies of antimicrobial agents such as biocides also depend upon their net charge and nature of contact surface (Campanac et al. 2002; Bridier et al. 2011). It implies that in background of diverse circumstances and contact surfaces used in food industry, routine cleaning regime with any random biocide, concentration, contact time and frequency may not be very much effective in prevention and eradication of biofilms. In this context, a previously study reported combination of SH and hydrogen peroxide to be effective in control and removal of P. aeruginosa biofilm developed on food contact surfaces (DeQueiroz and Day 2007). On the contrary, another study reported inefficiency of SH to eradicate biofilm from food contact surfaces (Byun et al. 2007). Apparently, selection of biocide for biofilm prevention and removal from food contact surfaces requires a serious strategy. A possible approach may involve comparative evaluation of common biocides in vogue for biofilm removal efficiencies. Such comparative studies in clinical background have helped in identifying biocides effective against biofilms of multidrug resistance Staphylococcus aureus and P. aeruginosa (Smith and Hunter 2008). Another study carried out with clinical isolates revealed that only 2 out of 12 biocides were effective against biofilm matrix of P. aeruginosa and S. aureus (Toté et al. 2010). The study also demanded guidelines for testing efficacy of biocides in biofilm removal.
Conversely in food environments, there is hardly any study on evaluation of different biocides for biofilm removal efficacy. Findings of such a study may be of considerable significance in planning better intervention strategies for ensuring food safety and quality. Hence in view of the gaps in current knowledge database, the present investigation was undertaken to evaluate biofilm formation potential of P. aeruginosa isolates from two dairy plants and compare common biocides for their antibiofilm efficacy.
Materials and methods
Sampling and test organism
Sampling, isolation and identification of the P. aeruginosa isolates has been described in details in earlier studies (Pagedar et al. 2011). Briefly, samples were collected by swabbing inner side of raw milk line (P1), pasteurizer inlet (P2) and outlet (P3) and milk tank (P4) of small scale (I) and commercial scale (II) dairy plants handling 50,000 and one million liters of milk per day, respectively. The sampling was done thrice in a year over the period of two years. After sample collection, cotton swabs were immediately immersed in tryptic soya broth (TSB) tubes and transported back to lab in an icebox for further analysis. The arbitrary nomenclature of the isolates was done according to their source, point of isolation and serial number during identification and/or screening. All isolates were identified and characterized biochemically and grown routinely in TSB. Whenever required, isolates counts were adjusted spectrophotometrically to obtain 108 cfu / ml followed by plating serial dilution onto plate count agar plates (PCA). All assays were carried out in triplicate and means were considered for subsequent analysis. Reagents and media were procured from HiMedia Labs, Mumbai, India unless mentioned otherwise.
Biofilm formation assay
Quantitative biofilm assay was performed as per previously published protocol (Pagedar et al. 2010) using a 96 well microtiter plate made of polystyrene (Costar, Cambridge, MA). Briefly, all isolates were grown overnight in TSB, inoculated at 1 % inoculum rate in 200 μl of the same medium and plates were incubated at 37 °C / 24 h. Quantitative estimation of biofilm formed was done using crystal violet staining method. Subsequently on the basis of optical density (A570), isolates were categorized as strong, moderate, weak and non-biofilm formers (Stepanovic et al. 2000).
Determination of inhibitory concentration of biocides against planktonic cells
Respective stock solutions of BC (106 ppm), SH (104 ppm), and ID (104 ppm) were appropriately diluted in Mueller Hinton Broth (MHB) tubes to obtain required range of concentrations (0 to 400 ppm in steps of 50 ppm for initial screening and then in steps of 5 ppm for determination of inhibitory concentration). Aliquots of 1 ml MHB containing different biocides concentrations, were inoculated (at 1 % inoculum rate) with overnight grown cultures and incubated at 37 °C / 24 h. Throughout the experiment, MHB tubes (without biocide) inoculated with respective cultures were used as positive controls, whereas un-inoculated MHB tubes (with biocides) as negative controls (Ac). After incubation, 200 μl aliquots (in triplicate) of respective cultures were transferred to a fresh 96 well-plate and optical density at 620 nm was measured using microplate reader (ECIL, Microscan, India). Inhibitory concentration end point was considered as lowest concentration of biocide at which absorbance of test (A) was less than or equal to that of un-inoculated control (Ac).
Determination of microbial biofilm eradication concentration of biocides
The efficiencies of biocides (50–900 ppm in steps of 50 for initial screening and then in steps of 5 ppm for determining MBEC) against biofilms of selected isolates were determined in terms of microbial biofilms eradication concentration (MBEC). To determine MBEC, contents of microtiter plate with 24 h old biofilm (107 cfu / 100 μl of MHB) were decanted and treated with biocides for contact time of 5, 15, 30, 60 min. The plates were then decanted, rinsed gently with sterile water and stained using crystal violet. The bound dye was re-solubilized in 33 % glacial acetic acid, absorbance of which was read as described in a previously published study (Pagedar et al. 2012). MBEC was considered as the concentration of biocide at which A < =Ac.
Statistical analysis
The arithmetic mean, standard deviation, significance (single factor ANOVA), was determined by using Microsoft Excel software (2007).
Results and discussion
Evaluation of biofilm formation potential
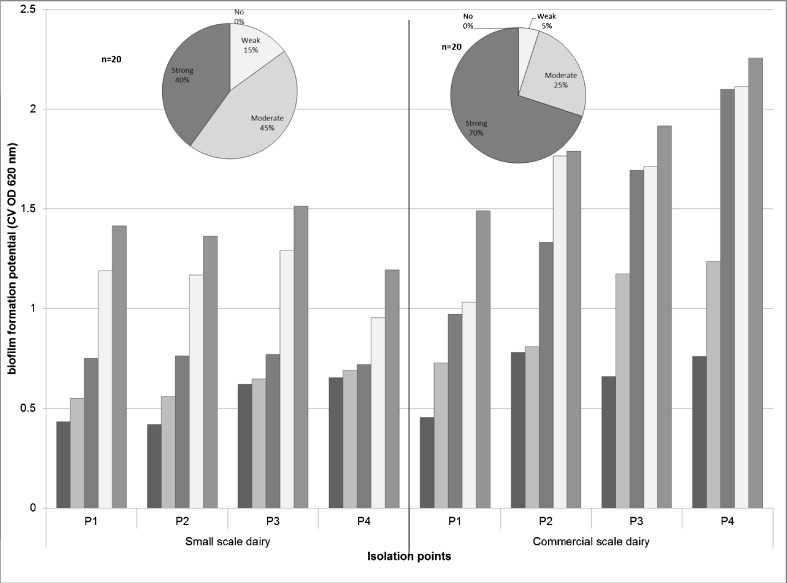
Forty isolates of P. aeruginosa were obtained from four isolation points (5 isolates from each point) of a small scale and commercial dairy. Biofilm formation was analyzed using microtiter plate assay and the isolates were categorized as “strong, moderate, weak and no biofilm formers” (as depicted in Fig. 1). Results revealed highly significant difference in BFP of isolates of two dairies and greater BFP of isolates from commercial dairy (P < 0.003). Moreover, greater proportion of strong biofilm formers was observed among isolates of commercial than small scale dairy. Almost 70 % of isolates obtained from commercial dairy were categorized as strong biofilm formers and 25 % as moderate and only 5 % as weak biofilm formers. On the other hand, the proportion of isolates obtained from small scale dairy was 40, 45 and 15 % for strong, moderate and weak biofilm formers, respectively. The results clearly indicate predominance of moderate biofilm formers among Pseudomonas isolates obtained from small scale dairy whereas those of strong biofilm formers in case of commercial scale dairy. Biofilm formation potential was independent of the point of isolation (P > 0.1), except for the bulk milk tank (post pasteurization point of isolation P4) (P < 0.05). These findings can be corroborated with those of our previous study, wherein almost similar trend was observed for BFP of Staphylococcus aureus from two dairies (Pagedar et al. 2010). It appears that irrespective of capacity and product profile, number and proportion of biofilm formers reflect upon cleaning regime of dairy plants. An inefficient cleaning procedure would not only deteriorate overall microbial quality of food products but also poses a substantial health hazard.
Fig. 1.
Biofilm formation potential of P. aeruginosa isolates obtained from small scale (I) and a commercial scale dairy (II) and categorization into strong, moderate, weak and non-biofilm formers (pie chart). P1 : Raw milk line; P2 : Pasteurizer inlet; P3 : Pasteurizer outlet; P4 : Milk tank
Evaluation of efficiency of BC, ID and SH against planktonic cells
Eight isolates i.e., one from each point of isolation (isolate exhibiting highest biofilm formation out of five) from both sources, exhibiting “strong” biofilm formation potential, were selected for this set of experiment. Selected isolates were confirmed to be representative of isolates from respective isolation points using box and whisker plots (data not shown). Our results show that all biocide were effective against planktonic cells of P. aeruginosa isolates at far less concentration than their biofilms (Table 1a–c). These findings are in line with those of a previous study that has reported adhered P. aeruginosa to be 300 times more resistant to biocides than planktonic cells (Sagripanti and Bonifacino 2000). Delayed or poor penetration of biocides across biofilm matrix, interaction with matrix components, and presence of organic materials have been recognized to be the major factors for poor efficiencies of biocides (Bridier et al. 2011). Moreover, P. aeruginosa cells have also been reported to alter cellular morphology, and quantum and spatial arrangement of extracellular matrix to resist various biocides (Dynes et al. 2009). Sub-lethal biocide concentrations, to which the biofilm inhabitants are subjected, may led to emergence and spread of antimicrobial resistance by developing adaptive and cross resistance and dissemination of persister or tolerant cells from biofilms.
Table 1.
Inhibitory concentrations of biocides (ppm) against planktonic cells and preformed biofilm (MBEC) of selected isolates of P. aeruginosa
| a: Benzalkonium chloride (BC) | |||||
| Isolates | Conc. of BC inhibiting Planktonic cells (ppm) | Conc. of BC to eradicate biofilm (MBEC in ppm) | |||
| 5 min | 15 min | 30 min | 60 min | ||
| I-P1-43 | 350 | >900 | 800 | 600 | 450 |
| I-P2-44 | 250 | >900 | 700 | 550 | 400 |
| I-P3-37 | 350 | >900 | 900 | 800 | 600 |
| I-P4-1 | 300 | >900 | 1,000 | 900 | 700 |
| II-P1-46 | 250 | >900 | 950 | 750 | 650 |
| II-P2-48 | 300 | >900 | 1,000 | 800 | 650 |
| II-P3-41 | 250 | >900 | 900 | 750 | 550 |
| II-P4-44 | 250 | >900 | 950 | 750 | 600 |
| b: Sodium Hypochlorite (SH) | |||||
| Isolates | Conc. of SH inhibiting Planktonic cells (ppm) | Conc. of SH to eradicate biofilm (MBEC in ppm) | |||
| 5 min | 15 min | 30 min | 60 min | ||
| I-P1-43 | 300 | 800 | 700 | 550 | 450 |
| I-P2-44 | 275 | 700 | 550 | 450 | 400 |
| I-P3-37 | 275 | 650 | 550 | 500 | 400 |
| I-P4-1 | 300 | 800 | 700 | 550 | 450 |
| II-P1-46 | 275 | 600 | 500 | 400 | 350 |
| II-P2-48 | 300 | 700 | 550 | 450 | 400 |
| II-P3-41 | 275 | 700 | 600 | 500 | 350 |
| II-P4-44 | 300 | 800 | 750 | 600 | 500 |
| c: Iodophore (ID) | |||||
| Isolates | Conc. of ID inhibiting Planktonic cells (ppm) | Conc. of ID to eradicate biofilm (MBEC in ppm) | |||
| 5 min | 15 min | 30 min | 60 min | ||
| I-P1-43 | 50 | 250 | 175 | 100 | 50 |
| I-P2-44 | 60 | 300 | 250 | 175 | 100 |
| I-P3-37 | 50 | 200 | 150 | 75 | 25 |
| I-P4-1 | 60 | 150 | 100 | 50 | 25 |
| II-P1-46 | 50 | 225 | 150 | 100 | 75 |
| II-P2-48 | 40 | 250 | 200 | 125 | 50 |
| II-P3-41 | 50 | 250 | 175 | 100 | 25 |
| II-P4-44 | 60 | 375 | 325 | 175 | 75 |
I : Small scale dairy, II: Commercial dairy, P1 : Raw milk line, P2 : Pasteurizer inlet, P3 : Pasteurizer outlet, P4 : Milk tank, MBEC: Microbial biofilms eradication concentration
Determination of minimum biofilm eradication concentration of BC, SH and ID
Minimum biofilm eradication concentration (MBEC) is determined either by incubating microorganism along with biocide or treating preformed biofilm with biocide for a specific contact time. The former method has been used to evaluate MBEC of bismuth based biocides, silver nitrate, EDTA and others which are of common use in hospitals environment (Leung et al. 2012). The latter method of MBEC determination has been explored more vigorously by several workers over a wide range of contact time duration (Smith and Hunter 2008; Bridier et al. 2011). In the current study, we evaluated MBEC using post-treatment format in order to mimic dairy CIP set up. Generally, contact time of 15 min is employed in dairy industry for biocide solution to run through dairy pipelines. However, the same contact time is not usually maintained for cleaning of silos, storage containers, bulk tanks and / or transportation tanks. Therefore in the current study, MBEC was evaluated over a time interval of 5, 15, 30, 60 min. Our findings indicate that biofilms of selected P. aeruginosa isolates were resistant to far higher concentrations of the biocides than recommended for routine applications (Table 1a–c). A trend of decreasing MBECs with extended contact time was observed but the decrease observed for ID (80 %) was more remarkable than BC (36 %) and SH (42 %) (Table 1a–c). For the contact time of 15 min, respective MBECs of BC, SH and ID were much higher than their recommended in-use concentrations (200 ppm for BC and SH, and 20 ppm for ID). Our findings show that all the three biocides were ineffective against preformed biofilm at recommended in-use concentrations. Previous studies with clinical isolates have also shown that most of the common biocides do not have any substantial effect on structural integrity of biofilm matrix. Our findings regarding the highest resistance of P. aeruginosa biofilms to BC are in harmony with those of a previously published study wherein up to 80 % of biofilm inhabitants were reported to survive recommended concentrations of in vogue biocides (Smith and Hunter 2008). Further supporting our observations, a comparative biocide efficacy study that included BC, SH, ID, isopropanol, peracetic acid and hydrogen peroxide found most of the biocides to be effective only against planktonic cells and not biofilms (Toté et al. 2010). In the current study performance of SH in eradication of P. aeruginosa biofilms was poorer than ID. However, contrary to our observations, a previous study has reported SH in combination with hydrogen peroxide to be very effective both against biofilm inhabitants and matrix on food contact surfaces (DeQueiroz and Day 2007; Toté et al. 2010). Another study on similar lines however, concluded otherwise and reported SH not to be much effective in removal of biofilms (Byun et al. 2007). The rationale for these contradictory observations may lie in different experimental factors such as selection of contact surface, time, origin of strains and possibility of strain’s pre exposure to the biocide. The findings of current study emphasize need of a systematic approach for selecting type and concentrations of biocides for application in food industry. Application of biocides at concentrations ineffective against biofilms will not only compromise food quality and safety but also help in emergence and spread of antimicrobial resistance.
Conclusion
The current study shows high prevalence of strong biofilm formers in commercial dairy, signifying importance of an efficient CIP regime. Inefficiency of in-use concentration of all the three biocides in removal of pre-formed biofilm warrants reconsideration over recommended in-use concentrations. Cleaning regimes in food industry need to be appropriately modified to target both removal and disinfection of biofilms. However, simply overuse/misuse of antimicrobials to control biofilms should be avoided as it may create selective pressure favoring emergence of adaptive and cross resistances and their transfer to non-resistant microflora. Such isolates in food processing scenarios may render routine cleaning regime less effective. These findings may be useful to plan better intervention strategies to ensure quality and safety in food industry.
References
- Bridier A, Dubois-Brissonnet F, Greub G, Thomas V, Briandet R. Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55(6):2648–2654. doi: 10.1128/AAC.01760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MW, Kim JH, Kim DH, Kim HJ, Jo C. Effects of irradiation and sodium hypochlorite on the micro-organisms attached to a commercial food container. Food Microbiol. 2007;24(5):544–548. doi: 10.1016/j.fm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Campanac C, Pineau L, Payard A, Baziard-Mouysset G, Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46(5):1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski RAN, Frank JF. Biofilm formation and control in food processing facilities. Compr Rev Food Sci F. 2003;2:23–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- DeQueiroz GA, Day DF. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. J App Microbiol. 2007;103(4):794–802. doi: 10.1111/j.1365-2672.2007.03299.x. [DOI] [PubMed] [Google Scholar]
- Dynes JJ, Lawrence JR, Korber DR, Swerhone GD, Leppard GG, Hitchcock AP. Morphological and biochemical changes in Pseudomonas fluorescens biofilms induced by sub-inhibitory exposure to antimicrobial agents. Can J Microbiol. 2009;55(2):163–178. doi: 10.1139/W08-109. [DOI] [PubMed] [Google Scholar]
- Franzetti L, Scarpellini M. Characterisation of Pseudomonas spp. isolated from foods. Ann Microbiol. 2007;57(1):39–47. doi: 10.1007/BF03175048. [DOI] [Google Scholar]
- Leung CY, Chan YC, Samaranayake LP, Seneviratne CJ. Biocide resistance of Candida and Escherichia coli biofilms is associated with higher antioxidative capacities. J Hosp Infect. 2012;81:79–86. doi: 10.1016/j.jhin.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Martin NH, Murphy SC, Ralyea RD, Wiedmann M, Boor KJ. When cheese gets the blues Pseudomonas fluorescens as the causative agent of cheese spoilage. J Dairy Sci. 2011;94(6):3176–3183. doi: 10.3168/jds.2011-4312. [DOI] [PubMed] [Google Scholar]
- Pagedar A, Singh J, Batish VK. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J Basic Microbiol. 2010;50(S1):S98–S106. doi: 10.1002/jobm.201000034. [DOI] [PubMed] [Google Scholar]
- Pagedar A, Singh J, Batish VK. Efflux mediated adaptive and cross resistance to ciprofloxacin and benzalkonium chloride in Pseudomonas aeruginosa of dairy origin. J Basic Microbiol. 2011;51(3):289–295. doi: 10.1002/jobm.201000292. [DOI] [PubMed] [Google Scholar]
- Pagedar A, Singh J, Batish VK. Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J Dairy Res. 2012;79(4):383–389. doi: 10.1017/S0022029912000295. [DOI] [PubMed] [Google Scholar]
- Sagripanti JL, Bonifacino A. Resistance of Pseudomonas aeruginosa to liquid disinfectants on contaminated surfaces before formation of biofilms. J AOAC Int. 2000;83(6):1415–1422. [PubMed] [Google Scholar]
- Smith K, Hunter IS. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966–973. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic MA. modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Toté K, Horemans T, Vanden Berghe D, Maes L, Cos P. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2010;76(10):3135–3142. doi: 10.1128/AEM.02095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst MA, Schuurmans JM, Smid MC, Koenders BB, Ter Kuile BH. De novo acquisition of resistance to three antibiotics by Escherichia coli. Microb Drug Resist. 2011;17:141–147. doi: 10.1089/mdr.2010.0101. [DOI] [PubMed] [Google Scholar]
- Van Tassell JA, Martin NH, Murphy SC, Wiedmann M, Boor KJ, Ivy RA. Evaluation of various selective media for the detection of Pseudomonas species in pasteurized milk. J Dairy Sci. 2012;95(3):1568–1574. doi: 10.3168/jds.2011-4958. [DOI] [PubMed] [Google Scholar]