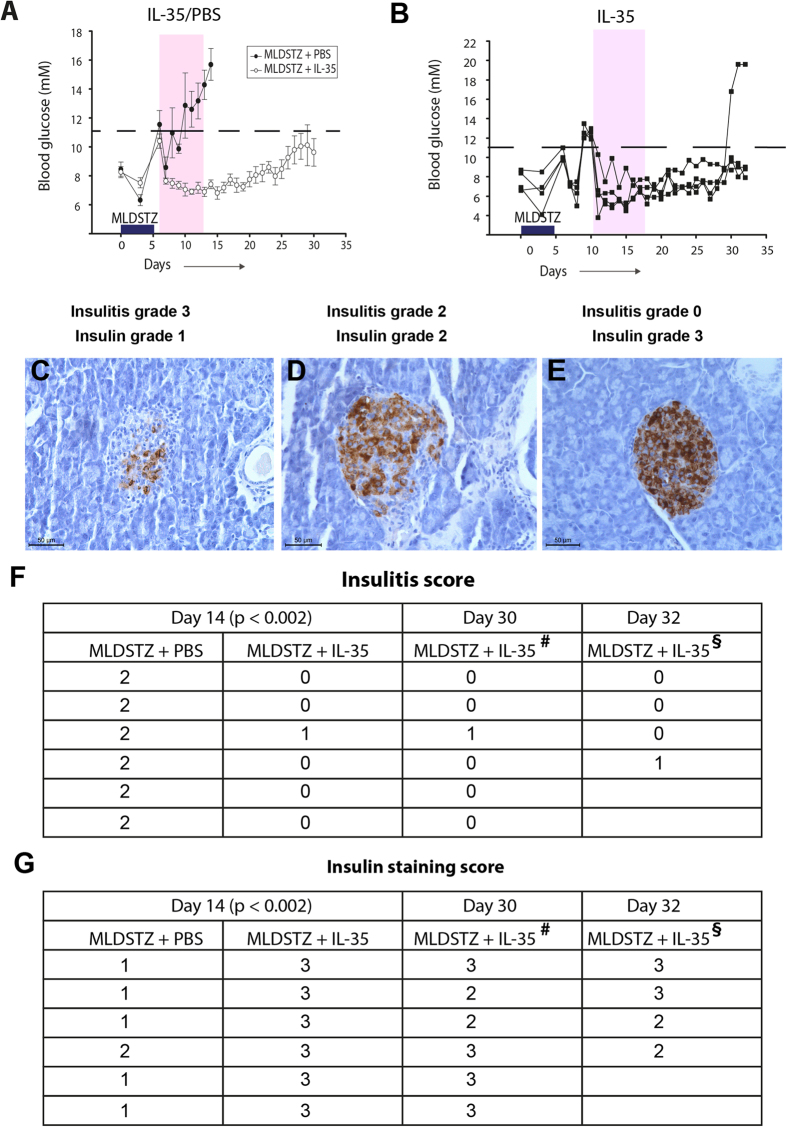
Figure 5. IL-35 prevents induction of MLDSTZ induced T1D and reverses established MLDSTZ induced T1D.
(A) MLDSTZ treated mice were injected with mouse recombinant IL-35 (0.75 μg/day, i.p.) or 200 μl PBS/day i.p. from day 6 for 8 days (shaded area). Six mice from the control group and six mice from the IL-35 treated group were sacrificed on day 14. After discontinuing IL-35 treatment, one mouse became hyperglycemic on day 26, while five mice remained normoglycemic. (B) MLDSTZ treated mice that had been hyperglycemic for two consecutive days (on day 10, after the first injection of STZ) were treated with mouse recombinant IL-35 for 8 days (shaded area) (n = 4 mice). Representative images of (C) insulitis grade 3 (>2/3 of the islet area infiltrated with mononuclear cells) and insulin grade 1 (<25% of insulin-positive cells). (D) Insulitis grade 2 (1/3-2/3 of the islet area infiltrated with mononuclear cells) and insulin grade 2 (25–50% insulin-positive cells). (E) Insulitis grade 0 (no infiltration in islets) and insulin grade 3 (>75% insulin-positive cells). (F) Haematoxylin and eosin stained sections of pancreata of IL-35 (0.75 μg daily, i.p.) or PBS (200 μl, i.p.) treated mice were analyzed for insulitis by scoring (0, 1, 2, 3 and 4) as described in the Methods section. (G) Insulin stained sections of pancreata of MLDSTZ + PBS or MLDSTZ + IL-35 treated mice were analyzed for insulin by scoring (0, 1, 2, and 3) as described in the Methods section. #MLDSTZ treated mice received IL-35 (0.75 μg daily, i.p.) for 8 days (starting from day 6 after the first injection of STZ). From day 14 (after the first injection of STZ) the IL-35 treatment was discontinued and mice were sacrificed on day 30 to analyze the morphology of pancreata. §MLDSTZ treated mice that had been hyperglycemic for two consecutive days (on day 10) were treated with mouse recombinant IL-35 (0.75 μg daily, i.p.) for 8 days as indicated in Fig. 4B. The mice were sacrificed on day 32. Mann-Whitney Rank Sum tests were performed for comparisons between MLDSTZ + PBS and MLDSTZ + IL-35 treated groups.