Abstract
ATP is omnipresent in biology and acts as an extracellular signaling molecule in mammals. Information regarding the signaling function of extracellular ATP in single-celled eukaryotes is lacking. Here, we explore the role of extracellular ATP in cell volume recovery during osmotic swelling in the amoeba Dictyostelium. Release of micromolar ATP could be detected during cell swelling and regulatory cell volume decrease (RVD) phases during hypotonic challenge. Scavenging ATP with apyrase caused profound cell swelling and loss of RVD. Apyrase-induced swelling could be rescued by 100 μM βγ-imidoATP. N-Ethylmalemide (NEM), an inhibitor of vesicular exocytosis, caused heightened cell swelling, loss of RVD, and inhibition of ATP release. Amoebas with impaired contractile vacuole (CV) fusion (drainin knockout [KO] cells) displayed increased swelling but intact ATP release. One hundred micromolar Gd3+ caused cell swelling while blocking any recovery by βγ-imidoATP. ATP release was 4-fold higher in the presence of Gd3+. Cell swelling was associated with an increase in intracellular nitric oxide (NO), with NO-scavenging agents causing cell swelling. Swelling-induced NO production was inhibited by both apyrase and Gd3+, while NO donors rescued apyrase- and Gd3+-induced swelling. These data suggest extracellular ATP released during cell swelling is an important signal that elicits RVD. Though the cell surface receptor for ATP in Dictyostelium remains elusive, we suggest ATP operates through a Gd3+-sensitive receptor that is coupled with intracellular NO production.
INTRODUCTION
The ability to control cell volume is an essential function for cell survival in the face of osmotic challenge. Perturbations in cell volume evoke wide-ranging signaling events leading to acute protective responses (e.g., rearrangement of the cytoskeleton) and longer-term adaptive responses (e.g., alterations in osmolyte transport and gene expression) (1). During acute swelling, cells can respond by a process of regulatory cell volume decrease (RVD). Under normal physiological conditions, mammalian cells are exposed to extracellular fluid osmolarity of approximately 285 mosmol, which is kept constant by normal body fluid homeostasis. Cell swelling often occurs as a consequence of changes to the intracellular composition of osmolytes, which results in intracellular hypotonicity and the influx of water. Compositional changes may occur during increased cellular transport or accumulation of nutrients or metabolic waste. Osmotically swollen mammalian cells release K+, Cl−, and nonessential organic osmolytes in an effort to reverse the flow of water by osmosis. In contrast to mammalian cells, free-living single eukaryotic cells can be subjected to rapid and harsh changes in the osmolarity of the extracellular environment. As a consequence, the majority of single-celled organisms have evolved a specialized organelle called the contractile vacuole, a bladder-like structure that plays a major role in extruding water from the cytoplasm and expelling it into the extracellular space (2).
ATP is a ubiquitous molecule used as energy currency by cells and as a substrate for protein phosphorylation inside the cell. In mammalian cells, extracellular ATP acts as a potent signaling molecule via activation of cell surface ionotropic (P2X) and metabotropic (P2Y) receptors. ATP release and signaling are involved in diverse physiological and pathophysiological events, including pain, inflammation, and control of blood vessel tone. The molecular mechanisms of ATP release in mammalian cells are also diverse, and further work is required to understand how cellular events are coupled with ATP release. ATP is released from mammalian cells when the cells are subjected to different types of mechanical force, including stretch (3, 4), flow (5), and shear (6) stresses. ATP is also released in response to osmotic swelling, acting as an early extracellular stress signal to initiate RVD via P2 receptor activation (7–9). Early studies demonstrated the presence of extracellular ATP in cultures of single-celled eukaryotes (10–12), but a role for extracellular ATP as a signal molecule in primitive organisms has not been defined. Parish and Weibel (10) published an early report demonstrating intracellular calcium responses evoked in the amoeba Dictyostelium by exogenous ATP. A more recent study by Ludlow et al. (13) also showed calcium response evoked by extracellular ATP. Both studies suggest the existence of cell surface receptors capable of responding to extracellular ATP, though the molecular basis for ATP reception and evidence for extracellular signaling by endogenous ATP are lacking. Therefore, we sought to investigate the role of extracellular ATP signaling during osmotic swelling in Dictyostelium.
MATERIALS AND METHODS
Cells and cell size measurement.
AX4 wild-type and drainin knockout (KO) AX4 Dictyostelium discoideum cells were cultured in shaking flasks containing HL5 medium (5 g/liter proteose peptone, 5 g/liter thiotone E peptone, 10 g/liter glucose, 5 g/liter yeast extract, 0.35 g/liter Na2HPO4, 0.35 g/liter KH2PO4, 0.05 g/liter dihydrostreptomycin, pH 6.5) at 22°C. Time-resolved measurement of changes in cell size were performed by right-angled light scattering (LS) at 600 nm using a Hitachi F2000 spectrophotometer. This photometric technique allows measurement of macroscopic cell size changes in populations of cells, where the intensity of scattered light correlates in a near-linear fashion with cell size (14).
Cells in culture were sedimented at 500 × g for 5 min at 22°C. The cells were resuspended at 2 × 106 cells/ml in HL5 medium or 2 mM HEPES-KOH (pH 7.2) for hypotonic challenge. The cells were continuously stirred in a quartz cuvette, and light at 600 nm was collected every 2 s. All compounds were injected manually. Experiments using the temperature-sensitive N-ethyl maleimide-sensitive factor (NSF) conditional-mutant strain were performed at 28°C.
ATP detection.
Samples (150 μl) were withdrawn from cell suspensions at regular intervals. The samples were spun immediately at 500 × g for 5 min at 4°C to produce a cell-free supernatant and to limit cell-dependent ATP breakdown. ATP was quantified by luciferase-luciferin assay as described previously (15).
NO assay.
NO2 and NO3 metabolites of nitric oxide (NO) were quantified by the modified Griess assay (16). Briefly, 2,3-diaminonaphthalene was reacted with samples under acidic conditions for 1 h at 37°C to form the fluorescent product 1-H-naphthotriazole. Accumulation of 1-H-naphthotriazole was measured using a fluorescence plate reader with excitation at 365 nm and emission at 450 nm.
Statistical analysis.
Hypothesis testing was performed by one-way analysis of variance (ANOVA).
RESULTS
Extracellular ATP is required for cell volume recovery following swelling in Dictyostelium amoebas.
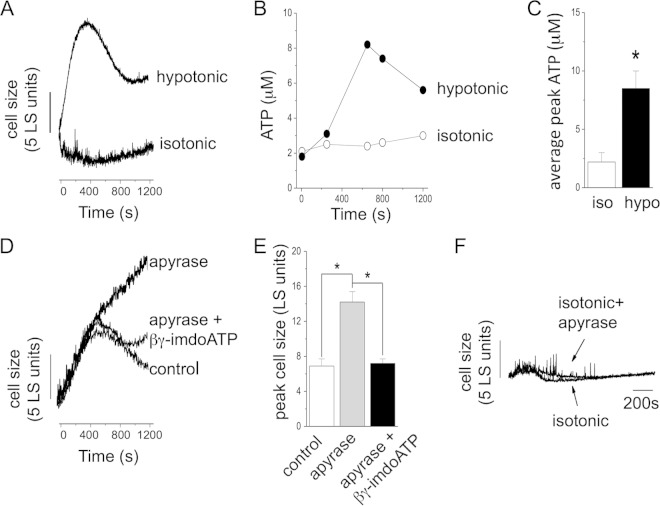
Dictyostelium amoebas exposed to hypotonic stress underwent cell swelling that peaked around 400 s postchallenge and was followed by a progressive cell volume recovery phase (Fig. 1A). At plateau, the average volume recovery was around 40% of peak (Fig. 1A). No significant changes in cell volume were observed under isotonic conditions (Fig. 1A). During cell swelling, extracellular ATP increased significantly, peaking at 8.1 ± 1.5 μM (n = 5) from a baseline of 2.2 ± 0.8 μM (n = 5) (Fig. 1C). No significant changes in extracellular ATP were observed under isotonic conditions (Fig. 1B). Application of apyrase to scavenge ATP during hypotonic challenge led to profound cellular swelling and loss of the cell volume recovery phase (Fig. 1D); detectable ATP was negligible following apyrase treatment (data not shown). Peak swelling observed in the presence of apyrase was 13.6 ± 1.5 light-scattering (LS) units (P < 0.05 versus control) compared to an average swelling of 6.8 ± 0.8 LS units in the absence of apyrase. The nonhydrolyzable ATP analogue βγ-imidoATP, but not ATP (data not shown), could rescue (97% ± 0.8%; n = 4; P < 0.01 versus the control) the volume recovery response in the presence of apyrase (Fig. 1D). Application of apyrase under isotonic conditions had no effect on cell size (Fig. 1E). Heat-inactivated apyrase also had no effect on cell size (data not shown). These data suggest that extracellular ATP is released during cell swelling and is important for cell volume recovery.
FIG 1.
Extracellular ATP released during cell swelling is required for regulatory cell volume decrease. (A) Representative LS experiment showing cell size changes in a suspension of Dictyostelium amoebas immediately after exposure to isotonic conditions or hypotonic challenge. (B) Representative plot showing extracellular ATP concentrations with time. (C) Mean peak extracellular ATP concentrations measured under isotonic (iso) and hypotonic (hypo) conditions (n = 5). (D) Representative trace showing the effect of apyrase (2 U/ml) on regulatory cell volume decrease during hypotonic challenge and rescue of a control response by βγ-imidoATP (100 μM). (E) Lack of effect of apyrase (2 U/ml) on cell size under isotonic conditions. *, P < 0.05. The error bars indicate standard errors of the means.
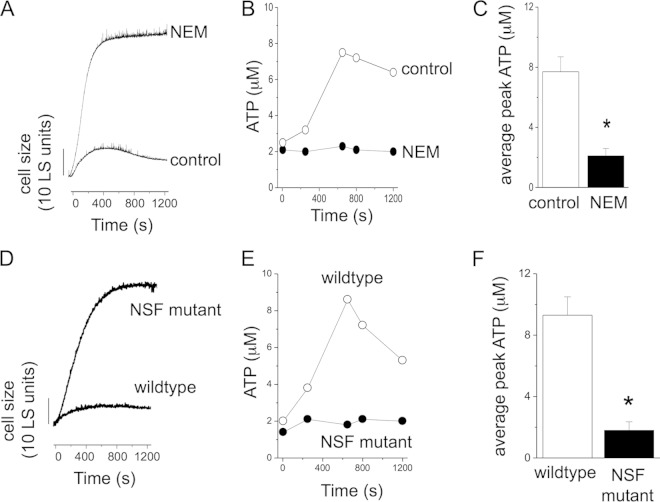
The contractile vacuole is the major osmoregulatory organelle in Dictyostelium and other protists. It serves to accumulate water during hypotonic stress and to release it via an atypical exocytosis event. We have previously demonstrated that the contractile vacuole system in Dictyostelium can accumulate ATP via a translocation mechanism with an unknown molecular basis (17). Furthermore, vesicular ATP release is one of numerous mechanisms proposed as modes of ATP secretion in mammalian cells (18). Therefore, we sought to employ N-ethylmaleimide (NEM), which blocks vesicular exocytosis and ATP release in mammalian cells (15). NEM caused profound swelling and loss of regulatory cell volume decrease under hypotonic conditions (Fig. 2A). Peak swelling in the presence of NEM was 46.5 ± 2.2 LS units (n = 6; P < 0.05 versus the control) compared to swelling in the absence of NEM (8.4 ± 1.2 LS units; n = 6). Interestingly, despite the heightened swelling caused by NEM, extracellular ATP accumulation was blocked (7.7 ± 1 μM [control] versus 2.1 ± 0.5 μM [NEM]; n = 6; P < 0.05) (Fig. 2B and C). In the presence of NEM, the peak ATP concentration was not significantly different from baseline, suggesting no ATP release (Fig. 2C). To further explore the role of exocytosis, we utilized a temperature-sensitive NSF conditional-mutant strain, which has been extensively studied and has impaired exocytosis (19, 20). NSF mutant cells swelled profoundly during hypotonic stress (Fig. 2D), with peak swelling of 40 ± 2.6 LS units (n = 6; P < 0.05 versus the control). Like NEM-treated wild-type cells, the NSF mutant also displayed impaired ATP secretion during hypotonic challenge (1.8 ± 0.2 μM [wild type] versus 9.1 ± 0.8 μM [NSF mutants]; n = 6; P < 0.05) (Fig. 2E and F). These data supported a vesicular exocytotic event as a possible route to ATP release and extracellular accumulation. In an effort to explore the role of contractile vacuole voiding in ATP release, we used drainin knockout amoebas, which display severely impaired membrane fusion of the contractile vacuole (21). Drainin knockout cells exhibited extensive swelling during hypotonic stress (25 ± 1.2 LS units versus 9.4 ± 0.8 LS units [control]; n = 5; P < 0.05) with total absence of any volume recovery phase (Fig. 3A), supporting a role for contractile membrane fusion in recovery from hypotonic swelling (21–23). Drainin knockout cells are null for the DDB_G0269130 gene, which encodes a rabGAP (21). In NEM-treated cells, extracellular ATP accumulation was still observed (Fig. 3B). Indeed, the peak concentrations of extracellular ATP were 2 times those in wild-type cells (Fig. 3C). Taken together, these data support a role for vesicular release of ATP during cell swelling but eliminate membrane fusion of the contractile vacuole as a potential source.
FIG 2.
Role of vesicular fusion in swelling-induced ATP release. (A) Representative LS experiment showing cell size changes in suspensions of wild-type Dictyostelium amoebas immediately after exposure to hypotonic challenge. The cells were pretreated with NEM (1 mM; 15 min) or not (control). (B) Representative plot showing extracellular ATP concentrations with time. (C) Mean peak extracellular ATP concentrations measured under hypotonic conditions with (NEM) and without (control) NEM pretreatment for wild-type cells (n = 6). (D and E) Representative LS experiments showing cell size changes in suspensions of wild-type Dictyostelium amoebas and temperature-sensitive NSF mutant cells. (F) Mean peak extracellular ATP concentrations measured under hypotonic conditions for wild-type and NSF mutant amoebas (n = 6). *, P < 0.05. The error bars indicate standard errors of the means.
FIG 3.
Role of contractile vacuole voiding in swelling-induced ATP release. (A) Representative LS experiment showing cell size changes in suspensions of wild-type Dictyostelium amoebas and drainin KO amoebas immediately after exposure to hypotonic challenge. (B) Representative plot showing extracellular ATP concentrations with time. (C) Mean peak extracellular ATP concentrations measured under hypotonic conditions for wild-type and drainin KO amoebas (n = 5). *, P < 0.05. The error bars indicate standard errors of the means.
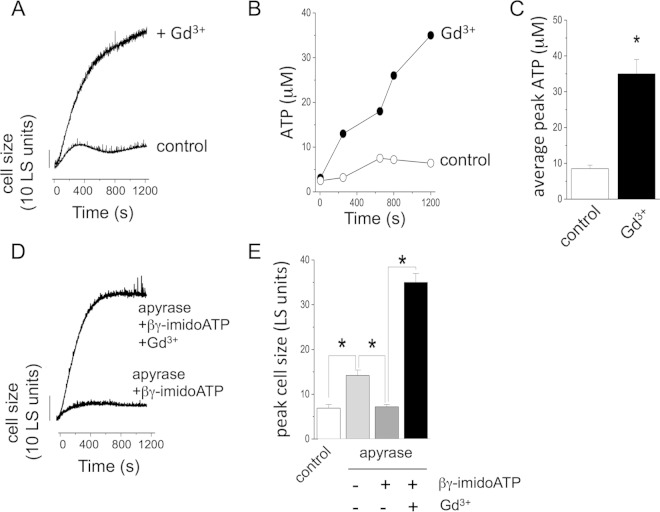
Previous work on ATP receptors in Dictyostelium has identified genes for five P2X receptor homologues (P2XA to P2XE) that encode ATP-activated ion channels (22, 23). In mammalian cells, P2X receptors are traditionally viewed as cell surface receptors for ATP, but our own work and that of others demonstrate an exclusively intracellular localization of Dictyostelium P2X receptors. Despite this, cellular responses to extracellular ATP have been reported (13), though the molecular basis for cell surface reception of ATP in Dictyostelium remains elusive. Ludlow et al. (13) reported that cellular responses to exogenous ATP were completely blocked by Gd3+. Therefore, we sought to examine the effect of micromolar Gd3+ on the cell-swelling response and ATP release. Exposure to Gd3+ mimicked the effect of apyrase, resulting in loss of the cell volume recovery phase and extensive cell swelling under hypotonic conditions (Fig. 4A). Unlike NEM, which uncoupled cell swelling from ATP release (Fig. 2B), ATP release was greatly heightened in the presence of Gd3+, with peak concentrations rising 4-fold (P < 0.05; n = 5) over control conditions (Fig. 4B and C). Interestingly, Gd3+ blocked any recovery from apyrase-induced swelling by addition of βγ-imidoATP (Fig. 4D and E). These data are suggestive of a Gd3+-sensitive ATP-dependent mechanism evoked during volume regulation under hypotonic stress.
FIG 4.
Gd3+ inhibits cell volume recovery from swelling and rescue by βγ-imidoATP during apyrase-induced swelling. (A) Representative LS experiment showing cell size changes in suspensions of wild-type Dictyostelium amoebas immediately after exposure to hypotonic challenge in the presence (+ Gd3+) or absence (control) of Gd3+ (100 μM). (B) Representative plot showing extracellular ATP concentrations with time. (C) Mean peak extracellular ATP concentrations measured under hypotonic conditions in the absence or presence of Gd3+ (100 μM) (n = 5). (D and E) Representative trace (D) and mean peak cell size (E) showing cell changes under hypotonic conditions. The cells were exposed to apyrase (2 U/ml) and βγ-imidoATP (100 μM) and to Gd3+ (100 μM) where indicated. The control values are from cells exposed to treatment (n = 5). *, P < 0.05. The error bars indicate standard errors of the means.
NO is produced by many cells during stress or trauma. To better understand possible signal transduction events that may result from ATP sensing during cell swelling, we investigated NO production as a feasible messenger. As for extracellular ATP, intracellular NO increased during cell swelling (Fig. 5A), reaching a steady-state phase several minutes after hypotonic challenge. Changes in NO levels were not observed under isotonic conditions (Fig. 5A). Next, we employed an NO scavenger to test whether the NO produced was important for the volume recovery process. Preincubation with the scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) resulted in loss of the cell volume recovery phase and profound swelling (85 ± 2.1 LS units [PTIO] versus 10.2 ± 0.6 LS units [control]; n = 5; P<0.05) (Fig. 5B), suggesting NO is required for volume recovery. Similar results were observed for the chemically unrelated NO scavenger sodium dimethyldithiocarbamate (SDTC) (data not shown). Application of the cell-impermeable NO scavenger hemoglobin had no effect (data not shown). To test for a requirement for either extracellular ATP or extracellular ATP sensing for NO production, we tested the effects of apyrase and Gd3+ on swelling-induced NO production. Both apyrase and Gd3+ ablated NO production during cell swelling (n = 5; P < 0.05) (Fig. 5C). In parallel experiments, we examined the ability of the NO donor sodium nitroprusside (SNP) to rescue swelling induced by either apyrase or Gd3+ in an effort to determine if NO lies downstream of ATP-sensing mechanisms at the cell surface. Strikingly, SNP could attenuate both apyrase- and Gd3+-induced swelling (Fig. 5D and E). SNP had no effect on cell volume under isotonic conditions (data not shown). These data demonstrate that NO donation can rescue the effects of apyrase and Gd3+, suggesting that NO acts downstream on pathways attenuated by apyrase and Gd3+.
FIG 5.
Nitric oxide production during hypotonic swelling is blocked by apyrase and Gd3+. (A) Relative quantification of cellular nitric oxide production in suspensions of Dictyostelium amoebas under hypotonic or isotonic conditions (n = 5). (B) Representative trace showing inhibition of cell volume recovery in cells treated with a nitric oxide-scavenging agent (PTIO) during hypotonic challenge. (C) Effect of apyrase (2 U/ml) or Gd3+ (100 μM) on nitric oxide production in cells under hypotonic stress (n = 5). (D and E) Representative traces showing effects of a nitric oxide donor (SNP; 500 μM) on swelling induced by apyrase (2 U/ml) (D) and Gd3+ (100 μM) (E). The error bars indicate standard errors of the means.
DISCUSSION
In this study, we propose that ATP released during hypotonic swelling of Dictyostelium amoebas operates as a stress signal through an unknown cell surface receptor to stimulate NO production and recover cell volume. This represents the first report of a role for endogenous extracellular ATP in a single-celled organism. P2X receptors for ATP have been cloned from several primitive species (22–29), including single cells, though the physiological role of ATP signaling in such organisms remains elusive. The subcellular distribution of P2X receptors in more primitive organisms also remains to be determined. It is highly likely that the P2X receptors identified in Dictyostelium do not serve as cell surface receptors for ATP due to their localization to the contractile vacuole (22, 23, 29). Though the contractile vacuole fuses with the plasma membrane during voiding of water, no mixing of the membranes occurs (30). This would eliminate vacuole fusion as a route for potential P2X receptor trafficking to the plasma membrane. Furthermore, intracellular calcium responses in Dictyostelium elicited by exogenous ATP application are unaffected by P2X receptor knockout (13). The well-curated genome information available for Dictyostelium also yields no information to suggest expression of P2Y receptor homologues (31). Recently, DORN1, which shows no homology with known P2X or P2Y receptors, was identified as a receptor for extracellular ATP in plants and is linked to stress signaling (32, 33). It is therefore possible that a novel receptor type mediates responsiveness to extracellular ATP in Dictyostelium.
The identification of cell surface ectonucleotidase-like activity in Dictyostelium provides a precedent for the existence of extracellular ATP (10), possibly in a signaling capacity. In addition, previous studies have demonstrated that Dictyostelium can condition growth medium with ATP (10). This suggests that Dictyostelium amoebas secrete ATP constitutively, as do some mammalian cells (15, 18). Our own bulk phase measurements suggest a relatively high basal level of extracellular ATP, between 2 and 4 μM. In this study, inhibition of vesicular secretion with NEM or by drainin knockout did not affect the level of basal ATP, though NEM strongly inhibited ATP release during swelling. Exocytosis of ATP-containing vesicles, such as lysosomes, has been shown to contribute to constitutive ATP secretion in mammalian cells (15), though this appears not to be the case for Dictyostelium. Our current study suggests that constitutively secreted ATP does not influence cell volume, as apyrase had no effect on cell size under isotonic conditions. The contractile vacuole of Dictyostelium harbors an ATP translocation mechanism with an unknown molecular basis and facilitates ATP accumulation within the vacuole lumen (17). Despite this, drainin knockout cells that have impaired contractile vacuole fusion exhibit heightened swelling but no inhibition of ATP release, strongly suggesting contractile vacuole voiding is not the source of ATP. As for Dictyostelium, Gd3+ inhibits RVD in various mammalian cells swollen by hypotonicity, including hepatocytes (34), neuronal cells (35), and erythrocytes (36). However, in mammalian cells, Gd3+ also blocks any swelling-induced ATP release (34, 37). This is in contrast to the heightened ATP release observed in Dictyostelium in the presence of Gd3+. In mammalian cells, the inhibitory action of Gd3+ on ATP release is reported to be through blockade of mechanosensitive receptors (7, 37, 38), which presumably integrate swelling-induced stretching of the plasma membrane and stimulate ATP release. Gd3+ also blocks receptor-mediated ATP release in mammalian cells (37). This suggests that ATP is released via a Gd3+-insensitive mechanism in Dictyostelium and that the increased ATP release occurs due to profound cell swelling observed in the presence of Gd3+. An alternative explanation for the Gd3+-induced swelling is that Gd3+ blocks sensing of extracellular ATP. This explanation is supported by the observation that Gd3+ blocks rescue by βγ-imidoATP during apyrase-induced swelling. βγ-ImidoATP can activate Dictyostelium P2X receptors (22), but as discussed above, the intracellular residency of P2X receptors makes them unlikely mediators. Ludlow et al. (13) demonstrated that Gd3+ could block calcium responses evoked by extracellular ATP in Dictyostelium. Our data support the presence of a Gd3+-sensitive cell surface receptor for ATP.
In this study, we demonstrate that intracellular NO increases during cell swelling. Experiments with an NO-scavenging agent suggest that the NO produced is important for the cell volume recovery process. Apyrase and Gd3+, which both block cell volume recovery, also block NO production during cell swelling. Moreover, NO donation can rescue cell volume recovery in the presence of apyrase or Gd3+. These data strongly suggest that NO production lies downstream of ATP sensing via a Gd3+ sensitivity receptor. In mammalian cells, such as red blood cells and vascular endothelium, NO is produced in response to mechanical stress (39, 40). There is also evidence that NO is produced by mammalian cells during osmotic and trauma-induced swelling (39, 40). Activation of the P2 receptor also stimulates NO production in various mammalian cell types (41–45). There are also a number of studies linking extracellular ATP to NO production in plant cells (46–48). In mammalian cells, NO is produced by nitric oxide synthase (NOS), though in plants, the identification of a mammalian-like NOS homologue remains elusive, despite the identification of NO-associated proteins (47). Nitrate reductases have been identified as enzymes responsible for NO production in plants (48). Evidence is also lacking for a homologue of mammalian NOS in Dictyostelium. The genome, however, does predict the existence of a homologue of NOS-interacting protein (47). In addition to the requirement for NO for cell volume regulation shown in this study, NO is known to control cellular aggregation and differentiation during multicellular development of Dictyostelium (49–51).
In summary, we have demonstrated that ATP is secreted during osmotic swelling in Dictyostelium via a NEM-sensitive mechanism. Based on the pharmacology of the cellular response, we suggest that extracellular ATP activates a Gd3+-sensitive receptor to increase intracellular NO, which in turn initiates cell volume recovery.
ACKNOWLEDGMENT
This work was funded by BBSRC grant BB/F023588 to S.J.F.
REFERENCES
- 1.Hoffmann EK, Pedersen SF. 2011. Cell volume homeostatic mechanisms: effectors and signalling pathways. Acta Physiol 202:465–485. doi: 10.1111/j.1748-1716.2010.02190.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen RD, Naitoh Y. 2002. Osmoregulation and contractile vacuoles of protozoa. Int Rev Cytol 215:351–394. doi: 10.1016/S0074-7696(02)15015-7. [DOI] [PubMed] [Google Scholar]
- 3.Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. 2009. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci 50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahara N, Ito S, Furuya K, Naruse K, Aso H, Kondo M, Sokabe M, Hasegawa Y. 2014. Real-time Imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol 51:772–782. doi: 10.1165/rcmb.2014-0008OC. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. 2011. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci 124:3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 6.Wan J, Ristenpart WD, Stone HA. 2008. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci U S A 105:16432–16437. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudreault F, Grygorczyk R. 2004. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouchs A, Ollivier H, Haond C, Roy S, Calves P, Pichavant-Rafini K. 2010. Activation of the MAPKs ERK1/2 by cell swelling in turbot hepatocytes. Biol Cell 102:447–456. doi: 10.1042/BC20090154. [DOI] [PubMed] [Google Scholar]
- 9.Espelt MV, de Tezanos Pinto F, Alvarez CL, Alberti GS, Incicco J, Leal Denis MF, Davio C, Schwarzbaum PJ. 2013. On the role of ATP release, ectoATPase activity, and extracellular ADP in the regulatory volume decrease of Huh-7 human hepatoma cells. Am J Physiol Cell Physiol 304:C1013–C1026. doi: 10.1152/ajpcell.00254.2012. [DOI] [PubMed] [Google Scholar]
- 10.Parish RW, Weibel M. 1980. Extracellular ATP, ecto-ATPase and calcium influx in Dictyostelium discoideum cells. FEBS Lett 118:263–266. doi: 10.1016/0014-5793(80)80234-1. [DOI] [PubMed] [Google Scholar]
- 11.Jakubowski H, Goldman E. 1988. Evidence for cooperation between cells during sporulation of the yeast Saccharomyces cerevisiae. Mol Cell Biol 8:5166–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyum R, Guidotti G. 1997. Glucose-dependent, cAMP-mediated ATP efflux from Saccharomyces cerevisiae. Microbiology 143:1901–1908. doi: 10.1099/00221287-143-6-1901. [DOI] [PubMed] [Google Scholar]
- 13.Ludlow MJ, Traynor D, Fisher PR, Ennion SJ. 2008. Purinergic-mediated Ca2+ influx in Dictyostelium discoideum. Cell Calcium 44:567–579. doi: 10.1016/j.ceca.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latimer P. 1979. Light scattering vs microscopy for measuring average cell size and shape. Biophys J 27:117–126. doi: 10.1016/S0006-3495(79)85206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivaramakrishnan V, Bidula S, Campwala H, Katikaneni D, Fountain SJ. 2012. Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes. J Cell Sci 125:4567–4575. doi: 10.1242/jcs.107318. [DOI] [PubMed] [Google Scholar]
- 16.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. 1993. A fluorometric assay for the measurement of nitrite in biological samples. Ann Biochem 214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 17.Sivaramakrishnan V, Fountain SJ. 2012. A mechanism of intracellular P2X receptor activation. J Biol Chem 287:28315–28326. doi: 10.1074/jbc.M112.372565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campwala H, Fountain SJ. 2013. Constitutive and agonist stimulated ATP secretion in leukocytes. Commun Integr Biol 6:e23631. doi: 10.4161/cib.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CRL, Bretscher MS. 2002. Cell polarity and locomotion, as well as endocytosis, depend on NSF. Development 129:4185–4192. [DOI] [PubMed] [Google Scholar]
- 20.Traynor D, Kay RR. 2007. Possible roles of the endocytic cycle in cell motility. J Cell Sci 120:2318–2327. doi: 10.1242/jcs.007732. [DOI] [PubMed] [Google Scholar]
- 21.Becker M, Matzner M, Gerisch G. 1999. Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. EMBO J 18:3305–3316. doi: 10.1093/emboj/18.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CRL, North RA. 2007. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature 448:200–203. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludlow MJ, Durai L, Ennion SJ. 2009. Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J Biol Chem 284:35227–35239. doi: 10.1074/jbc.M109.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agboh KC, Webb TE, Evans RJ, Ennion SJ. 2004. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem 279:41650–41657. doi: 10.1074/jbc.M408203200. [DOI] [PubMed] [Google Scholar]
- 25.Bavan S, Straub VA, Blaxter ML, Ennion SJ. 2009. A P2X receptor from the tardigrade species Hypsibius dujardini with fast kinetics and sensitivity to zinc and copper. BMC Evol Biol 9:17. doi: 10.1186/1471-2148-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavan S, Farmer L, Singh SK, Straub VA, Guerrero FD, Ennion SJ. 2011. The penultimate arginine of the carboxyl terminus determines slow desensitization in a P2X receptor from the cattle tick Boophilus microplus. Mol Pharmacol 79:776–785. doi: 10.1124/mol.110.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fountain SJ, Cao L, Young MT, North RA. 2008. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem 283:15122–15126. doi: 10.1074/jbc.M801512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fountain SJ. 2013. Primitive ATP-activated P2X receptors: discovery, function and pharmacology. Front Cell Neurosci 7:247. doi: 10.3389/fncel.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baines A, Parkinson K, Sim JA, Bragg L, Thompson CR, North RA. 2013. Functional properties of five Dictyostelium discoideum P2X receptors. J Biol Chem 288:20992–21000. doi: 10.1074/jbc.M112.445346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuser J. 2006. Evidence for recycling of contractile vacuole membrane during osmoregulation: a tribute to Gunther Gerisch. Eur J Cell Biol 85:859–871. doi: 10.1016/j.ejcb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Fountain SJ, Burnstock G. 2009. An evolutionary history of P2X receptors. Purinergic Signal 5:269–272. doi: 10.1007/s11302-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Tanaka K, Nguyen CT, Stacey G. 2014. Extracellular ATP is a central signaling molecule in plant stress responses. Curr Opin Plant Biol 20:82–87. doi: 10.1016/j.pbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G. 2014. Identification of a plant receptor for extracellular ATP. Science 343:290–294. doi: 10.1126/science.343.6168.290. [DOI] [PubMed] [Google Scholar]
- 34.Roman RM, Feranchak AP, Davison AK, Schwiebert EM, Fitz JG. 1999. Evidence for Gd(3+) inhibition of membrane ATP permeability and purinergic signaling. Am J Physiol 277:G1222–G1230. [DOI] [PubMed] [Google Scholar]
- 35.Lippmann BJ, Yang R, Barnett DW, Misler S. 1995. Pharmacology of volume regulation following hypotonicity-induced cell swelling in clonal N1E115 neuroblastoma cells. Brain Res 686:29–36. doi: 10.1016/0006-8993(95)00447-X. [DOI] [PubMed] [Google Scholar]
- 36.Bergeron LJ, Stever AJ, Light DB. 1996. Potassium conductance activated during regulatory volume decrease by mudpuppy red blood cells. Am J Physiol 270:R801–R810. [DOI] [PubMed] [Google Scholar]
- 37.Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. 2000. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl-conductances in murine C127 cells. J Physiol 523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stout CE, Costantin JL, Naus CC, Charles AC. 2002. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 39.Ulker P, Meiselman HJ, Baskurt OK. 2010. Nitric oxide generation in red blood cells induced by mechanical stress. Clin Hemorheol Microcirc 45:169–175. doi: 10.3233/CH-2010-1293. [DOI] [PubMed] [Google Scholar]
- 40.Kolluru GK, Sinha S, Majumder S, Muley A, Siamwala JH, Gupta R, Chatterjee S. 2010. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of eNOS: a basis for shear stress mediated angiogenesis. Nitric Oxide 22:304–315. doi: 10.1016/j.niox.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Liu R, Pittner J, Persson AE. 2002. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13:2688–2696. doi: 10.1097/01.ASN.0000033275.17169.67. [DOI] [PubMed] [Google Scholar]
- 42.Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. 2008. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol 67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- 43.Shalev M, Staerman F, Allain H, Lobel B, Saiag B. 1999. Stimulation of P2y purinoceptors induces, via nitric oxide production, endothelium-dependent relaxation of human isolated corpus cavernosum. J Urol 161:955–959. doi: 10.1016/S0022-5347(01)61828-7. [DOI] [PubMed] [Google Scholar]
- 44.Cabral PD, Hong NJ, Garvin JL. 2012. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol 303:F194–F200. doi: 10.1152/ajprenal.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintas C, Pinho D, Pereira C, Saraiva L, Goncalves J, Queiroz G. 2014. Microglia P2Y6 receptors mediate nitric oxide release and astrocyte apoptosis. J Neuroinflammation 11:141. doi: 10.1186/s12974-014-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ. 2009. Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot 60:2129–2138. doi: 10.1093/jxb/erp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gas E, Flores-Perez U, Sauret-Gueto S, Rodriguez-Concepcion M. 2009. Hunting for plant nitric oxide synthase provides new evidence of a central role for plastids in nitric oxide metabolism. Plant Cell 21:18–23. doi: 10.1105/tpc.108.065243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. 2002. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110. doi: 10.1093/jexbot/53.366.103. [DOI] [PubMed] [Google Scholar]
- 49.Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, van Driessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R, Hauser H, James K, Quiles M, Madan Babu M, Saito T, Buchrieser C, Wardroper A, Felder M, Thangavelu M, Johnson D, Knights A, Loulseged H, Mungall K, Oliver K, Price C, Quail MA, Urushihara H, Hernandez J, Rabbinowitsch E, Steffen D, Sanders M, Ma J, Kohara Y, Sharp S, Simmonds M, Spiegler S, Tivey A, Sugano S, White B, Walker D, Woodward J, Winckler T, Tanaka Y, Shaulsky G, Schleicher M, Weinstock G, Rosenthal A, Cox EC, Chisholm RL, Gibbs R, Loomis WF, Platzer M, Kay RR, Williams J, Dear PH, Noegel AA, Barrell B, Kuspa A. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature 435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao Y, Howlett A, Klein C. 1992. Nitric oxide-releasing compounds inhibit Dictyostelium discoideum aggregation without altering cGMP production. FEBS Lett 314:49–52. doi: 10.1016/0014-5793(92)81459-Y. [DOI] [PubMed] [Google Scholar]
- 51.Tao YP, Misko TP, Howlett AC, Klein C. 1997. Nitric oxide, an endogenous regulator of Dictyostelium discoideum differentiation. Development 124:3587–3595. [DOI] [PubMed] [Google Scholar]