FIG 12.
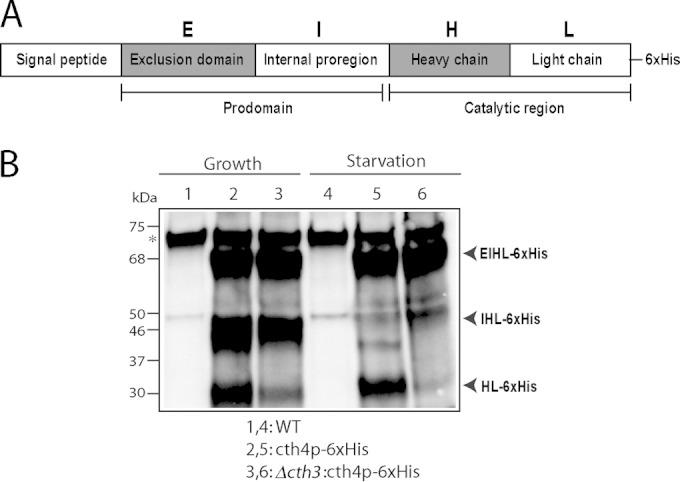
Cth4p undergoes Cth3p-dependent processing (A) Proposed organization of T. thermophila Cth4p, based on established structures of cathepsin C homologs in other eukaryotes. S, signal sequence; E, exclusion prodomain; I, internal proregion; H, heavy chain; L, light chain. The catalytic domains are the heavy and light chains. (B) Processed forms of Cth4p in T. thermophila. Cth4p-6×His expression was induced for 2 h in wild-type or Δcth3 cultures. Cells were induced in either growth or starvation media, by adding 1 or 0.1 μg/ml of CdCl2, respectively. Cell lysates (3 × 104 cell equivalents/lane) were separated by SDS-PAGE and Western blots were probed with anti-His MAb. Three major His-tagged species are present, and their tentative relationship with the domains indicated in panel A is indicated. Particularly in starvation, the large majority of Cth4p in Δcth3 cells remains in an unprocessed form that is predicted to be inactive. Note that starvation is a state in which transcription of Cth3p and Cth4p, as well as processing of proGrl, are induced.
