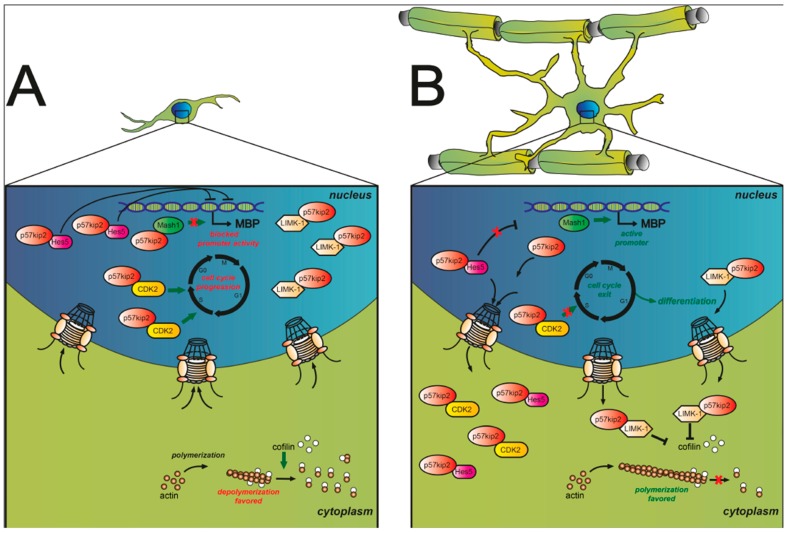
Figure 2.
(A) In early stages of oligodendroglial precursor cell (OPC) differentiation p57kip2 is predominantly located in nuclei. Kinase binding partners such as LIM Domain Kinase 1 (LIMK-1) and CDK2 appear to colocalize with p57kip2. Nuclear presence of CDK2 results in maintenance of cell cycle progression, while nuclear LIMK-1 is unable to inhibit cytoplasmic cofilin, a negative regulator of actin filament turnover. As a result actin depolymerization is enhanced. Moreover, interactions of p57kip2 with the transcription factors Ascl1/Mash1 and Hes5 appear to control target gene transcription. Upon p57kip2 binding Ascl1/Mash1’s transcriptional activity was found to be blocked while Hes5 nuclear accumulation appears to be enforced by p57kip2 where it is supposed to interfere with myelin gene expression; (B) Mature oligodendroglial cells exhibit high cytoplasmic levels of p57kip2. Nuclear export of p57kip2 leads to dissociation from its binding partner Ascl1/Mash1 and results in myelin gene activation by this transcription factor. Furthermore, CDK2 and Hes5 translocate from the nucleus to the cytoplasm along with p57kip2 and are thus rendered functionally inactive. In addition, enhanced cytoplasmic levels of LIMK-1 can affect actin filament turnover. Cytoplasmic LIMK-1 is phosphorylating cofilin, which in turn interferes with its capacity to depolymerize actin filaments. Subsequently, this process of cytoplasmic trapping of inhibitory components by the p57kip2 protein, allows terminal differentiation to proceed.