Table 1.
Glycosylation using glycosyl donors with various ester-protecting groups at C2
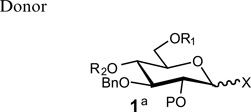 |
Acceptors | |
|---|---|---|
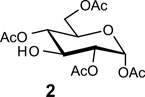 |
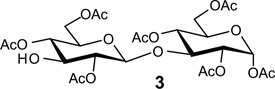 |
|
| 1a P=Ac X= O-trichloroacetimidateb | 84% (α:β 1:1) | NA |
| 1b P=Bz X= O-trichloroacetimidateb | NA | 86% (α only) |
| 1c P = ClCH2CO X = O-trichloroacetimidateb | NA | 85% (α only) |
| 1d P=CH3OCO X = O-trichloroacetimidateb | 85% (α:β 1:1) | 83% (α only) |
| 1e P = (CH3)3CCO X = O-trichloroacetimidateb | 80% (α only) | 81% (α:β 2:3) |
| 1f P = (CH3)3CCO X = SPhc | NA | 82% (α:β 1:3) |
Compound 1a–e, R1 = Ac, R2 = Ac; compound 1f, R1:R2 = CHPh.
Activation with TMSOTf.
Activation with NIS/AgOTf.
