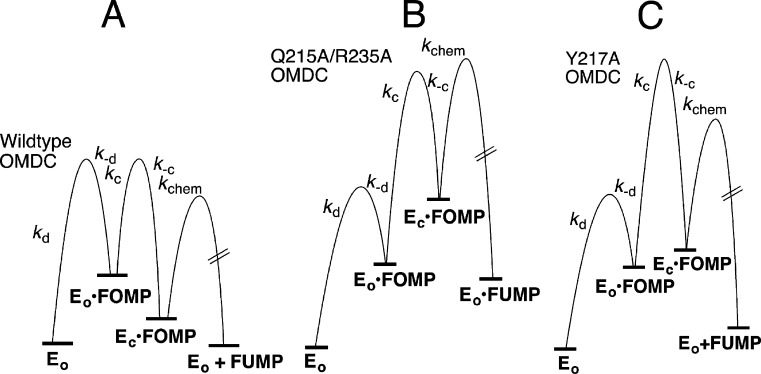
Figure 5.
Free energy profiles for ScOMPDC-catalyzed decarboxylation of FOMP (Scheme 5), drawn for reactions at [S] ≪ Km using the kinetic parameters from Table 2. (A) Decarboxylation of FOMP catalyzed by wild-type OMPDC, which shows (a) the similar barriers to partitioning of EO·FOMP between dissociation of OMP and the enzyme conformational change to form EC·FOMP (k–d ≈ kc),27 (b) thermodynamically favorable conversion of EO·FOMP to the EC·FOMP caged complex,19 and (c) the ≈3.5 kcal/mol difference [RT ln(390/1.1) (Scheme 2)] between the barriers for formation of EC·FOMP and decarboxylation of EC·FOMP. (B) Decarboxylation of FOMP catalyzed by the Q215A/R235A mutant of ScOMPDC. This mutation results in a 106-fold decrease in Kc for loop closure compared with that of wild-type ScOMPDC, but in little change in intrinsic barrier Λ for loop closure (Table 2). The decarboxylation step is rate-determining because k–c > kchem. (C) Decarboxylation of FOMP catalyzed by the Y217A mutant of ScOMPDC. This mutation results in a 2000-fold decrease in Kc for loop closure and a large increase in intrinsic barrier Λ for slow loop closure (Table 2), so that loop closure is rate-determining for this decarboxylation reaction.