Abstract
The purpose of this study is to compare the efficacy of a single administration of dexamethasone (DEX) on day 1 against DEX administration on days 1–3 in combination with palonosetron (PALO), a second-generation 5-HT3 receptor antagonist, for chemotherapy-induced nausea and vomiting (CINV) in non-anthracycline and cyclophosphamide (AC) moderately-emetogenic chemotherapy (MEC). This phase III trial was conducted with a multi-center, randomized, open-label, non-inferiority design. Patients who received non-AC MEC as an initial chemotherapy were randomly assigned to either a group administered PALO (0.75 mg, i.v.) and DEX (9.9 mg, i.v.) prior to chemotherapy (study treatment group), or a group administered additional DEX (8 mg, i.v. or p.o.) on days 2–3 (control group). The primary endpoint was complete response (CR) rate. The CR rate difference was estimated by logistic regression with allocation factors as covariates. The non-inferiority margin was set at −15% (study treatment group − control group). From April 2011 to March 2013, 305 patients who received non-AC MEC were randomly allocated to one of two study groups. Overall, the CR rate was 66.2% in the study treatment group (N = 151) and 63.6% in the control group (N = 154). PALO plus DEX day 1 was non-inferior to PALO plus DEX days 1–3 (difference, 2.5%; 95% confidence interval [CI]: −7.8%–12.8%; P-value for non-inferiority test = 0.0004). There were no differences between the two groups in terms of complete control rate (64.9 vs 61.7%) and total control rate (49.7% vs 47.4%). Anti-emetic DEX administration on days 2–3 may be eliminated when used in combination with PALO in patients receiving non-AC MEC.
Keywords: Chemotherapy-induced nausea and vomiting, dexamethasone, moderately-emetogenic chemotherapy, palonosetron, phase III
Appropriate management of adverse events due to chemotherapy is a key factor in continuation of chemotherapy for malignant tumors. Of all chemotherapy-related adverse events, emesis is the most common form of non-hematological toxicity. Although there are differences in severity, 70–80% of patients experience emesis during chemotherapy.1 If chemotherapy-induced nausea and vomiting (CINV) is not controlled, patients may lose their motivation to continue treatment, resulting in refusal of chemotherapy or reduced compliance that ultimately shortens survival. CINV also negatively impacts the quality of life (QOL) of cancer patients.2,3 Thus, suppressing CINV is a crucial aspect of successful chemotherapy.
The Perugia Antiemetic Consensus Guideline Meeting established standards for classifying chemotherapy agents into four groups according to emetic risk: highly-emetogenic chemotherapy (HEC), moderately-emetogenic chemotherapy (MEC), low-emetogenic chemotherapy (LEC) and those with minimal risk.4 These classifications are currently used in many guidelines. MEC indicates risk in 30–90% of patients. Combined 5-hydroxytryptamine 3 (5-HT3) receptor antagonists and dexamethasone (DEX) are recommended for prophylaxis of acute (within 24 h of chemotherapy administration) emesis, while DEX alone is recommended for control of delayed emesis (more than 24 h post-administration).5
Palonosetron (PALO) is a novel 5-HT3 receptor antagonist that has exhibited high selective affinity for the 5-HT3 receptor in various experimental models. Mean half-life of the drug was approximately 40 h after both i.v. and p.o. administration in a clinical pharmacological study of PALO in healthy adults, or 4–10 times longer than other 5-HT3 receptor antagonists.6 This long half-life and high affinity for 5-HT3 receptors are the main benefits of PALO. A phase III double-blind comparative trial was performed in Japan with PALO and granisetron in cancer patients receiving HEC; this study demonstrated non-inferiority for PALO in terms of the complete response (CR) rate for acute vomiting and superiority for PALO in terms of the CR rate for delayed vomiting.7
This suggests the possibility of eliminating the day 2 and 3 DEX doses to control delayed CINV when using a PALO combination during chemotherapy. Previous reports have shown non-inferiority of day 1 PALO and day 1 DEX against day 1 PALO and day 1–3 DEX in patients receiving MEC (including anthracycline and cyclophosphamide [AC]).8,9
Given the above, the present study was performed to determine whether day 2 and 3 DEX doses to control delayed vomiting could be eliminated in non-AC MEC in combination with PALO. Demonstration of non-inferiority of anti-emetic effects after elimination of day 2 and 3 DEX doses would help prevent overtreatment with DEX and help alleviate associated adverse events and other forms of patient burden, ultimately contributing to increased patient QOL.
Patients and Methods
Study design
The present study was a multicenter, open-label, non-inferiority, randomized comparative phase III trial, with study centers consisting of 18 locations in Hokkaido, Japan. The enrollment phase lasted from April 2011 to March 2013. Enrollment subjects were randomly allocated to one of two groups (control or study treatment group) following confirmation that they met all inclusion criteria and did not meet any exclusion criteria. Randomization was performed according to the minimization method using the four factors of: study center, chemotherapy regimen (oxaliplatin [L-OHP]-based, irinotecan [CPT-11]-based, carboplatin [CBDCA]-based or other), sex (male or female) and age (under 55 years of age or 55 years and older).
In accordance with Ethical Guidelines for Clinical Studies, clinical research ethical review boards at all institutions reviewed and approved the study prior to study initiation. The study was also registered with the UMIN Clinical Trials Registry (UMIN000009403). Finally, in accordance with Japanese ethical guidelines on clinical research and at the request of the ethical review board of Hokkaido University Hospital, clinical research insurance was obtained to compensate subjects in case of damage to health.
Subjects
Written informed consent was obtained from all subjects prior to enrollment. Primary inclusion criteria were age of 20 years or older, diagnosis of malignant tumor, no history of chemotherapy (patients who had received LEC were permitted), planned administration of non-AC MEC, and sufficient marrow, renal and kidney function to tolerate chemotherapy. Primary exclusion criteria were a history of grade 2 or higher nausea prior to enrollment, previous PALO use, planned administration of MEC on multiple sequential days, and planned administration of cisplatin (CDDP) regardless of dose.
Outcomes
The objective of the current study is to assess the optimum dosage method for DEX when used in combination with PALO. The primary endpoint was overall CR rate, while secondary endpoints were CR rate (acute and delayed phases), complete control (CC) rate (overall, acute, and delayed) and frequency/severity of adverse events. The total control (TC) rate was not prescribed in the protocol, but we assessed it as a post hoc analysis. “Overall” is defined as up to 120 h from chemotherapy administration, “acute” is defined as the phase up to 24 h post-administration, and “delayed” is defined as the phase 24–120 h post-administration.
Gastrointestinal (GI) symptom diaries were used to evaluate efficacy. Patients received a complete explanation of the GI symptom diaries, and were then requested to make entries every 24 h up to 120 h post-administration. The diaries enabled entry of the number of vomiting events and severity of nausea. Nausea was evaluated according to a four-step Likert scale.
The CR rate is defined as the proportion of subjects in the analysis set with no emetic events and no anti-emetic measures. The CC rate is the proportion of subjects with no emetic events, no anti-emetic measures, and either no nausea or mild nausea. Finally, TC rate is the proportion of subjects with no emetic events, no anti-emetic measures and no nausea. Adverse events were evaluated according to Common Terminology Criteria for Adverse Events v. 4.0. Exacerbation of symptoms by one grade level or higher compared to before initiation of the study is considered an adverse event; incidence rates of adverse events for which a causal relationship with the study treatment could not be denied were calculated for the analysis population.
Treatments
The following treatments were performed for each group. The control group received 9.9 mg DEX i.v. followed by 0.75 mg PALO i.v. prior to MEC administration. Counting the day of MEC treatment as day 1, DEX was administered on days 2 and 3 either p.o. (8 mg) or i.v. (6.6 mg). The study treatment group received 9.9 mg DEX i.v., followed by 0.75 mg PALO i.v. prior to MEC. Thereafter, MEC only was administered. We held medical interviews when collecting patients’ diaries, and asked the patients whether they had taken the steroid medication on days 2 and 3. The study protocol allowed the use of 5-HT3 receptor antagonists as antiemetics, corticosteroids (excluding dexamethasone), antidopaminergic agents, phenothiazine tranquilizers, antihistamines, and benzodiazepines as rescue drugs, which were equally available to all subjects. It prohibited the use of NK1 receptor antagonists, SSRI and SNRI.
Statistical analysis and sample size calculation
The primary purpose of the present study was to evaluate the non-inferiority of the study treatment group compared to the control group in subjects receiving non-AC MEC. The primary analysis was performed by applying a logistic regression model on the full analysis set using all allocation factors excluding study center (chemotherapy treatment, sex and age) as covariates to estimate the difference in the overall CR rate between the two groups (study treatment group − control group) and its two-sided Wald-type 95% confidence interval (CI). The margin of non-inferiority was set at 15%, and non-inferiority was assessed by checking whether the lower bound of the 95% CI of the difference of the overall CR rate included −15%. The margin of 15% corresponds to approximately 1.25 based on the risk ratio scale because the expected CR rate was set at 70%. The non-inferiority margin of 1.25 is not very large in general. In addition, previous similar studies8,9 also set the non-inferiority margin at 15%. Therefore, we have assumed that the non-inferiority margin of 15% is acceptable in this setting. This was a non-inferiority study, so a one-sided significance level of 2.5% was used. Treatment-by-subgroup interaction was assessed using the Gail–Simon test.10 All efficacy outcomes were assessed for the intention-to-treat population, which included all randomized patients except those who did not receive any treatment at all or who were ineligible after randomization. Subjects who have no data after randomization were excluded from the safety population.
The target sample size was determined in the following manner. In a Japanese dose-response trial 11 of PALO in MEC patients, the overall CR rate for the PALO 0.75-mg group was 69.6%. The number of subjects required per group was calculated at 147 assuming expected CR rates of 70% for both groups, a non-inferiority margin of 15%, statistical power of 80%, a one-sided alpha error of 2.5% and, because dynamic allocation was used, a multiple correlation coefficient of 0 for allocation factors used in the logistic regression modeling and groups. Therefore, the target sample size was set at 150 per group, or a total of 300, to allow for some dropouts.
Results
Patient characteristics and baseline demographics
Figure1 shows the CONSORT diagram for the study. Out of 308 subjects enrolled, 154 were allocated randomly to the control group and the study treatment group. Of these, 305 were included in the efficacy analysis population. Of the three subjects excluded from the efficacy analysis population, 2 withdrew prior to protocol treatments while 1 was discovered not to meet inclusion requirements. The safety analysis population consisted of 298 subjects.
Fig 1.
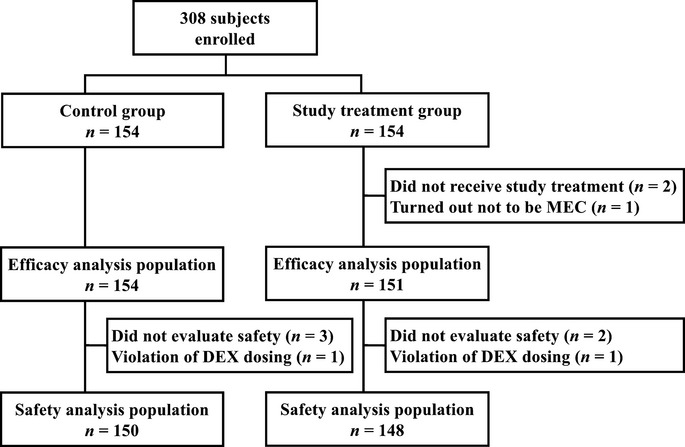
CONSORT diagram.
No significant differences were observed in the 308 randomly-allocated subjects in terms of allocation factors (Table1). Most subjects were 55 years of age or older (85.0%). While most subjects used an L-OHP-based chemotherapy regimen (72.8%), only 12.1% used a CBDCA-based regimen.
Table 1.
Baseline demographics and clinical characteristics
| Control group† (n = 154) | Study treatment group‡ (n = 151) | |
|---|---|---|
| Gender, n (%) | ||
| Female | 67 (43.5) | 65 (43.0) |
| Male | 87 (56.5) | 86 (57.0) |
| Age, n (%) | ||
| <55 years | 22 (14.3) | 24 (15.9) |
| ≥55 years | 132 (85.7) | 127 (84.1) |
| Mean (min–max) | 64.0 (23–88) | 64.1 (34–85) |
| Alcohol consumption within 180 days of enrollment, n (%) | ||
| Yes | 68 (44.2) | 70 (46.4) |
| No | 80 (51.9) | 77 (51.0) |
| Unknown | 6 (3.9) | 4 (2.6) |
| Chemotherapy regimen, n (%) | ||
| Oxaliplatin-based | 112 (72.7) | 110 (72.8) |
| Irinotecan-based | 20 (13.0) | 21 (13.9) |
| Carboplatin-based | 19 (12.3) | 18 (11.9) |
| Other | 3 (1.9) | 2 (1.3) |
†Control group: Palonosetron day 1+ dexamethasone days 1–3. ‡Study treatment group: Palonosetron day 1+ dexamethasone day 1.
Efficacy
The primary endpoint of the overall CR rate for the 305 subjects in the efficacy analysis population was 63.6% (98/154) in the control group and 66.2% (100/151) in the study treatment group. The difference in CR rates between the two groups according to the logistic regression model with allocation factors as covariates was 2.5% (95% CI: −7.8% to 12.8%; P-value for non-inferiority test = 0.0004), demonstrating non-inferiority of the study treatment group. There were no large differences between the two groups according to phase (Table2). Subgroup analysis of the CR rate according to age (below 55 years or 55 years and above), sex (male or female) or chemotherapy (L-OHP-based, CPT-11-based, CBDCA-based or other) revealed a beneficial trend for the control group, with −25.8% for below 55 years of age (P-value for interaction = 0.081) in spite of no statistically significant treatment-by-subgroup interaction in this study (Table3).
Table 2.
Efficacy
| Control group† (n = 154) | Study treatment group‡ (n = 151) | Difference between arms (95% CI) | |
|---|---|---|---|
| Complete response§ rate, n (%) | |||
| Overall (0–120 h) | 98 (63.6) | 100 (66.2) | 2.5% (−7.8–12.8%) |
| Acute (0–24 h) | 142 (92.2) | 141 (93.4) | 1.2% (−4.6–7.0%) |
| Delayed (24–120 h) | 100 (64.9) | 101 (66.9) | 1.9% (−8.4–12.2%) |
| Complete control¶ rate, n (%) | |||
| Overall (0–120 h) | 95 (61.7) | 98 (64.9) | 3.2% (−7.2–13.5%) |
| Acute (0–24 h) | 142 (92.2) | 141 (93.4) | 1.2% (−4.6–7.0%) |
| Delayed (24–120 h) | 97 (63.0) | 99 (65.6) | 2.6% (−7.9–12.9%) |
| Total control†† rate, n (%) | |||
| Overall (0–120 h) | 73 (47.4) | 75 (49.7) | 2.3% (−8.5–13.1%) |
| Acute (0–24 h) | 134 (87.0) | 129 (85.4) | −1.6% (−9.3–6.2%) |
| Delayed (24–120 h) | 74 (48.1) | 76 (50.3) | 2.3% (−8.9–13.5%) |
†Control group: Palonosetron day 1+ dexamethasone days 1–3. ‡Study treatment group: Palonosetron day 1+ dexamethasone day 1. §Complete response: No emetic events and no anti-emetic measures (nausea allowed). ¶Complete control: No emetic events, no anti-emetic measures, and no nausea or mild nausea. ††Total control: No emetic events, no anti-emetic measures and no nausea. CI, confidence interval.
Table 3.
Subgroup analysis of complete response rates by allocation factors
| Control group† (n = 154) | Study treatment group‡ (n = 151) | Difference between arms (95% CI) | Interaction test | |
|---|---|---|---|---|
| Age, n (%) | ||||
| Under 55 years | 13 (59.1) | 8 (33.3) | −25.8% (−53.7–2.1%) | 0.081 |
| 55 years or older | 85 (64.4) | 92 (72.4) | 8.1% (−3.2–19.3%) | |
| Sex, n (%) | ||||
| Male | 62 (71.3) | 65 (75.6) | 4.3% (−8.8–17.5%) | 0.500 |
| Female | 36 (53.7) | 35 (53.9) | 0.1% (−16.9–17.1%) | |
| Chemotherapy regimen, n (%) | ||||
| Oxaliplatin-based | 71 (63.4) | 76 (69.1) | 5.7% (−6.7–18.1%) | 0.351 |
| Irinotecan-based | 13 (65.0) | 14 (66.7) | 1.7% (−27.4–30.7%) | |
| Other | 14 (63.6) | 10 (50.0) | −13.6% (−43.4–16.1%) | |
†Control group: Palonosetron day 1+ dexamethasone days 1–3; ‡Study treatment group: Palonosetron day 1+ dexamethasone day 1. CI, confidence interval.
Analysis on the influence of subject background factors showed adjusted odds ratio of sex (female vs male) 0.57 (95% CI: 0.33–1.00; P = 0.049), per 10-year increments 1.44 (95% CI: 1.12–1.85; P = 0.005), alcohol (yes vs no) 1.56 (95% CI: 0.88–2.76; P = 0.125), complication (yes vs no) 0.53 (95% CI: 0.28–0.98; P = 0.042), regimen (CPT-11 vs others) 1.47 (95% CI: 0.56–3.87; P = 0.652) and regimen (L-OHP vs others) 1.50 (95% CI = 0.73–3.07; P = 0.462).
Adverse events
Proportions of incidence of adverse events for the 298 subjects in the safety analysis population were 30.0% in the control group and 23.0% in the study treatment group, resulting in no significant difference between the two groups. Primary adverse events observed were constipation (control group, 14.0%; study treatment group, 12.2%), hiccups (control group, 8.0%; study treatment group, 2.7%), anorexia (control group, 5.8%; study treatment group, 3.6%), and elevated ALT (control group, 5.4%; study treatment group, 5.4%) (Table4). The incidence rate of adverse events that might have been attributable to the steroid medication, such as abdominal pain and hiccups, tended to be lower in the day 1 group, but no statistical significance was observed.
Table 4.
Treatment-related adverse events (AE)
| n (%) | Control group† (n = 150) | Study treatment group‡ (n = 148) | ||||
|---|---|---|---|---|---|---|
| Any Grade | ≥Grade 2 | ≥Grade 3 | Any Grade | ≥Grade 2 | ≥Grade 3 | |
| All AE | 45 (30) | 9 (6) | 1 (0.7) | 34 (23) | 7 (4.7) | 0 (0) |
| Elevated AST | 7 (4.7) | 1 (0.7) | 0 (0) | 7 (4.8) | 1 (0.7) | 0 (0) |
| Elevated ALT | 8 (5.4) | 1 (0.7) | 1 (0.7) | 8 (5.4) | 1 (0.7) | 0 (0) |
| Constipation | 21 (14) | 1 (0.7) | 0 (0) | 18 (12.2) | 2 (1.4) | 0 (0) |
| Abdominal pain | 6 (4) | 0 (0) | 0 (0) | 2 (1.4) | 0 (0) | 0 (0) |
| Hiccups | 12 (8) | 4 (2.7) | 0 (0) | 4 (2.7) | 1 (0.7) | 0 (0) |
| Anorexia | 8 (5.8) | 3 (2.2) | 0 (0) | 5 (3.6) | 1 (0.7) | 0 (0) |
†Control group: palonosetron day 1+ dexamethasone days 1–3. ‡Study treatment group: palonosetron day 1+ dexamethasone day 1.
Discussion
This study successfully demonstrated non-inferiority of DEX administration on day 1 only compared to days 1–3 when used in combination with PALO during non-AC MEC. DEX is widely used for CINV in clinical situations; a meta-analysis of 32 studies covering 5613 subjects resulted in a risk ratio of 1.26 for the acute phase and 1.29 for the delayed phase when comparing the DEX group to the no-treatment group, demonstrating anti-emetic efficacy of the drug.12 However, one draw-back of this analysis is the almost complete lack of non-AC, non-CDDP regimens covered. Furthermore, there is currently no evidence nor consensus on optimal dosages or treatment periods of DEX for delayed emesis.12 Although DEX itself is considered to have mild adverse reactions overall, they include hyperglycaemia, drowsiness, headaches, feeling hot, chills, oral dryness, diarrhea, euphoria, lassitude, upper abdominal pain and rashes. DEX should be avoided in patients with a history of inadequately controlled diabetes mellitus or GI ulcers.
PALO is the only 5-HT3 receptor antagonist that has been demonstrated to be effective against delayed emesis. It is known to have a longer plasma half-life than other 5-HT3 receptor antagonists, but this alone is insufficient to explain efficacy in the delayed phase. Delayed emesis is known as primarily mediated by substance P, whose action on the medullary vomiting center is mediated by the neurokinin 1 receptor. Therefore, inhibiting substance P is key to inhibiting delayed emesis. Basic research on whether or not PALO inhibits substance P has shown that, unlike other 5-HT3 receptor antagonists, the drug does exert inhibitory effects on various reactions with which substance P is involved,(13) a result supporting the findings of the present study.
Eliminating DEX from all anti-emetic therapy using PALO in non-AC MEC is not recommended. Although DEX does cause the adverse events described above, subgroup analysis of the current study warranted that eliminating DEX doses on days 2 and 3 tends to reduce CR rates for subjects under 55 years of age.
Analysis on the influence of subject background factors showed that odds ratio per 10-year increments 1.44 (95% CI: 1.12–1.85) and the age was the significant factor that influenced CR rates. Younger patients are generally considered to be at increased risk of CINV. As similar results were observed in this study, we wish to stress the importance of anti-emetic therapy for younger patients.
Of all the subjects receiving non-L-OHP-based and non-CPT-11-based regimens in this study, 88.1% received carboplatin-based treatment. This drug induces emesis at a higher rate than other MEC,14 and is also considered to have greater prolonged effects in the delayed phase. Emetic risk may also be highly dependent on dosage (area under the curve), so care is required when administering anti-emetic therapy.
However, our study has a limitation. This study is not a double-blind trial but an open label one. We do not deny the possibility that bias may occur. As the results of our study, however, are comparable to those of similar studies8,9 that were conducted abroad, we believe that our results are reproducible and its influence on the results is small. However, it should be remembered that the DEX-related results described here were obtained in combination with PALO, so DEX administration should not be eliminated in combination with other 5-HT3 receptor antagonists. However, the study did demonstrate the usefulness of 0.75 mg PALO as well in the delayed phase of non-AC MEC, results which should help alleviate patient burden associated with excessive treatment of DEX and improve QOL.
Acknowledgments
We thank all of the patients, their families and the institutions involved in this study. The study was supported by the Hokkaido Supportive Care Oncology Project – An Expert Group initiative, HOPE Group.
Disclosure Statement
Dr Yoshito Komatsu has received fees for promotional materials from Bayer Yakuhin, Taiho Pharmaceutical, Takeda Pharmaceutical, Chugai Pharmaceutical and Pfizer Japan.
References
- Aapro MS. Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manag. 2007;3:1009–20. [PMC free article] [PubMed] [Google Scholar]
- Lindley CM, Hirsch JD, O’Neill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1:331–40. doi: 10.1007/BF00434947. [DOI] [PubMed] [Google Scholar]
- Morita S, Kobayashi K, Eguchi K, et al. Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non-small cell lung cancer: application of a general linear model. Jpn J Clin Oncol. 2003;33:470–6. doi: 10.1093/jjco/hyg083. [DOI] [PubMed] [Google Scholar]
- Roila F, Hesketh PJ, Herrstedt J. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17:20–8. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- Siddiqui MA, Scott LJ. Palonosetron. Drugs. 2004;64:1125–32. doi: 10.2165/00003495-200464100-00006. ; discussion 1133–4. [DOI] [PubMed] [Google Scholar]
- Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–24. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- Aapro M, Fabi A, Nolè F, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–8. doi: 10.1093/annonc/mdp584. [DOI] [PubMed] [Google Scholar]
- Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer. 2011;19:1217–25. doi: 10.1007/s00520-010-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail M, Simon R. Tests for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–72. [PubMed] [Google Scholar]
- Segawa Y, Aogi K, Inoue K, et al. A phase II dose-ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide-based chemotherapy. Ann Oncol. 2009;20:1874–80. doi: 10.1093/annonc/mdp243. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: a meta-analysis of randomized evidence. J Clin Oncol. 2000;18:3409–22. doi: 10.1200/JCO.2000.18.19.3409. [DOI] [PubMed] [Google Scholar]
- Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010;335:362–8. doi: 10.1124/jpet.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist. 1999;4:191–6. [PubMed] [Google Scholar]


