Fig 3.
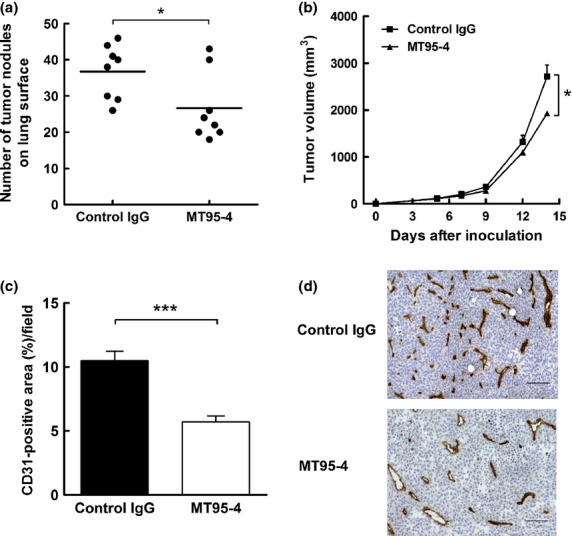
Effects of MT95-4 on tumor progression in the tail vein metastasis model and subcutaneous tumor model using APN-B16 cells. Mice were administered MT95-4 or control IgG (1 mg/kg) i.p. twice a week. (a) Evaluation of the number of lung surface nodules in the tail vein metastasis model following injection of APN-B16 cells. The number of tumor nodules on the lung surface was counted 21 days after injection of APN-B16 cells. Each bar represents the mean number of nodules from eight mice per group. $P < 0.05. (b) Evaluation of tumor volume in a subcutaneous tumor model using APN-B16 cells. The sizes of subcutaneous tumors were measured twice a week for 2 weeks after inoculation of APN-B16 cells. The data represent the means (± SEM) from eight mice per group. $P < 0.05. (c) Evaluation of angiogenesis in subcutaneous tumors derived from APN-B16 cells. The area of CD31-positive vessels was measured. Data represent the means (± SEM) from eight mice per group. $$$P < 0.01. (d) Immunohistochemical staining for CD31 in a subcutaneous tumor. Scale bar, 100 μm.
