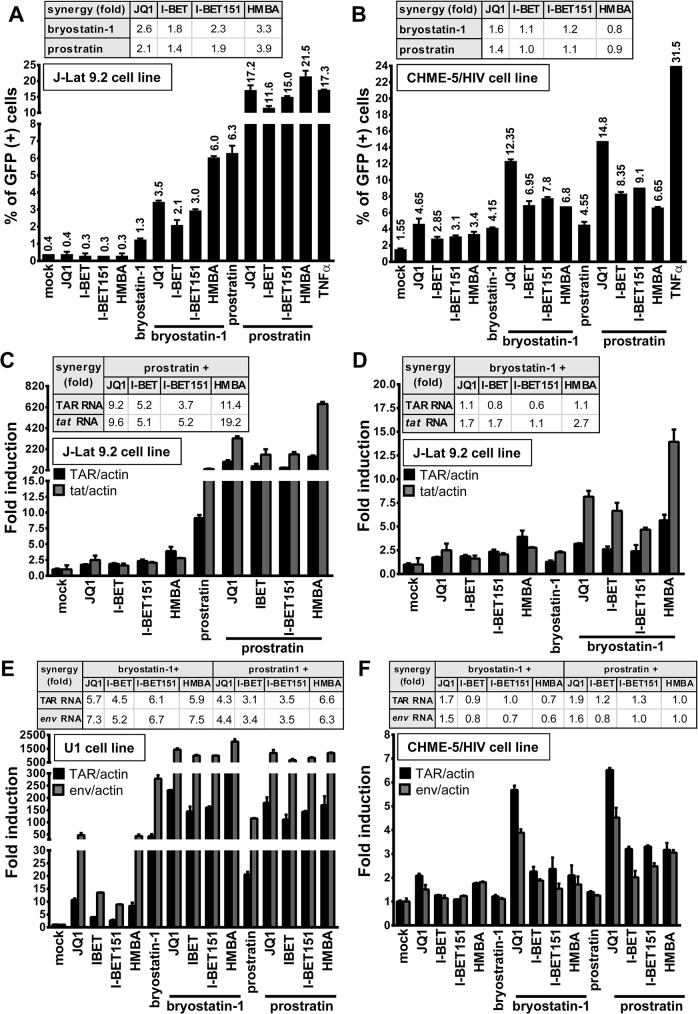
Fig 2. PKC agonist+BETi/HMBA combined treatments increase HIV-1 expression in a higher proportion of cells than the drug alone and synergistically enhance viral transcription.
The J-Lat 9.2 cells (panel A) or CHME-5/HIV microglial cells (panel B) harbor latent HIV-1 provirus containing gfp gene. The cells were mock-treated, treated with JQ1 (0.5μM), I-BET (0.5μM), I-BET151 (0.5μM), HMBA (5mM), bryostatin-1 (10nM) and prostratin (2.5μM) alone or in combination as indicated. At 24 hours post-treatment, cells were analyzed by flow cytometry to quantify the proportion of cells expressing GFP. Means and standard errors of the means from duplicate samples are indicated. One representative experiment from three is represented. For each combinatory treatment, the fold-synergy was calculated by dividing the effect observed after co-treatments by the sum of the effects obtained from individual treatments. Panels C-F. Measurement of initiated and elongated HIV-1 transcripts following drug treatment. Total RNA was extracted from J-Lat 9.2 (panels C and D), U1 (panel E), CHME-5/HIV (panel F) cells which were mock-treated or treated with BETi, HMBA, bryostatin-1 and prostratin for 24 hours at concentrations described in Fig 2A and 2B. Initiated (primers TAR) or elongated (primers tat or env) transcripts were quantified by quantitative real-time RT-PCR. Values were normalized using β-actin gene primers and were presented as fold inductions relative to the values measured in mock-treated cells, which were arbitrarily set at a value of 1. Means and standard errors of the means from duplicate samples are indicated. One representative experiment from two is represented. For each combinatory treatment, the fold-synergy was calculated by dividing the effect observed after co-treatments by the sum of the effects after the individual treatments.