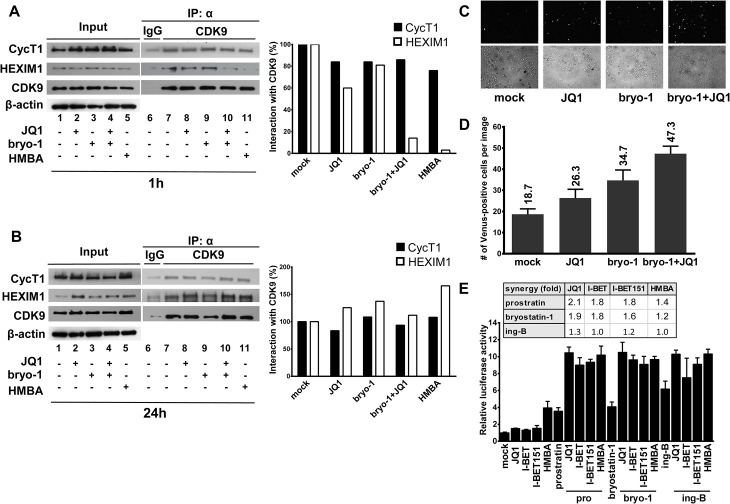
Fig 8. PKC agonists synergize with BETi in releasing active P-TEFb.
Jurkat cells were mock-treated, treated with JQ1 (0.25μM), bryostatin-1 (5nM) alone or in combination for 1 hour (Panel A) or 24 hours (Panel B). Nuclear extracts were prepared from treated cells and subjected to immunoprecipitations (IP) with an anti-CDK9 antibody or the control IgG. The complexes were immunodetected for the presence of CycT1 and HEXIM-1 by Western blotting (right panels). Input controls for CDK9, CycT1 and HEXIM1 are presented (left panels). Levels of β-actin were measured to control protein loading. Panels A and B. Histograms represent quantification of band intensities normalized to CDK9 levels in the IP and then normalized to mock-treated condition. Panel C. HeLa cells expressing YC.P-TEFb and VN.CTD were left untreated or were treated as indicated for 1 hour. Venus-positive cells were detected by fluorescence microscopy (upper panels). Bright-field images were also taken (lower panels). Panel D. HeLa cells expressing YC.P-TEFb and VN.CTD were treated as outlined in C and Venus-positive cells were counted and averaged from three different areas. Error bars represent differences between counts of Venus-positive cells from the randomly chosen fields under the microscope. Panel E. Hela cells were transfected with the Hex1(-104)Luc reporter plasmid. At 24 hours post-transfection, cells were mock-treated or treated with the different compounds as indicated. Luciferase activities in cell extracts were measured 24 hours after drug treatments and reported as fold increases over the activity observed in mock-treated condition (transfection of the reporter plasmid without drug treatment) and arbitrarily set at a value of 1. An experiment performed in duplicate representative of two independent experiments is shown.