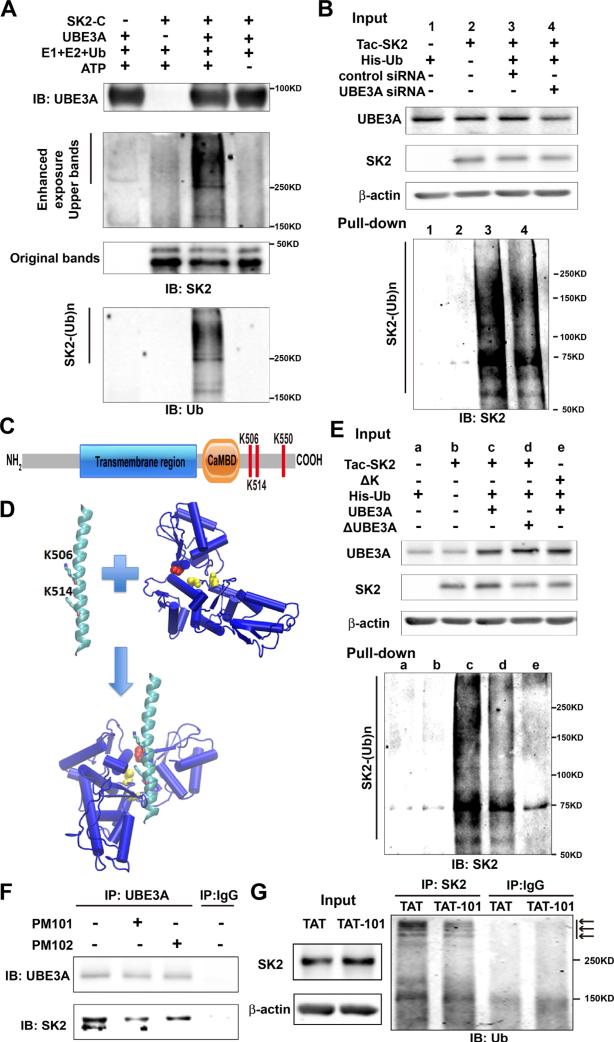
Figure 3. UBE3A ubiquitinates SK2 in the C-terminal domain.
(A) In vitro ubiquitination of SK2 by recombinant UBE3A. Reaction products were analyzed by Western blot with UBE3A (upper panel), SK2 (middle panel), or ubiquitin (Ub, lower panel) antibodies. Note that ubiquitin and SK2 dually labelled high molecular bands (SK2-(Ub)n) are present only when all reaction elements and ATP were added.
(B) siRNA knock-down of UBE3A in COS-1 cells reduces SK2 ubiquitination. COS-1 cells were incubated with UBE3A siRNA or scrambled control siRNA 24 h before transfection with Tac-SK2 and His-ubiquitin. Twenty-four h later, ubiquitinated proteins were isolated by Co2+-affinity chromatography. Levels of ubiquitinated Tac-SK2 protein (SK2-(Ub)n, lower panel) were determined by Western blot. Levels of input proteins were also evaluated by Western blot probed with UBE3A, SK2, and β-actin antibodies (Upper panel).
(C) Candidate UBE3A ubiquitination sites (K506/K514/K550) within SK2 C-terminus. CaMBD: calmodulin-binding domain.
(D) Docking of SK2 C-terminal helix (PDB ID 2PNV, chain A 488 to 526) into UBE3A (PDB ID 1D5F). K506 and K514 in the SK2 C-terminal helix are shown in stick. C604 and C737 in the larger N-terminal lobe of UBE3A are shown in yellow balls, and catalytic C820 in the smaller C-terminal lobe is shown in red.
(E) K506A/K514A/K550A (ΔK) mutations in SK2 block UBE3A-mediated SK2 ubiquitination. UBE3A over-expression enhances and K506A/K514A/K550A mutations block SK2 ubiquitination in COS-1 cells. His-tagged ubiquitinated proteins in cells co-transfected with either Tac-SK2 plus UBE3A or ΔUBE3A or ΔK plus UBE3A were precipitated using Talon resin and probed with anti-SK2 antibody (lower panel). Ubiquitinated SK2 are labeled with “SK2-(Ub)n”. Upper panel, input of UBE3A, SK2, and β-actin.
(F) Binding of SK2 with UBE3A is interrupted by PM101 and PM102 peptides. Hippocampal P2 fractions from WT mice were incubated in the presence or absence of PM101 or PM102 (50 μM) peptides followed by immunoprecipitation with either anti-UBE3A antibodies or control IgG. Western blots were performed with anti-UBE3A or anti-SK2 antibodies. Levels of SK2 co-immunoprecipitated with UBE3A were decreased by peptides PM101 or PM102.
(G) Binding of SK2 with UBE3A and its ubiquitination in vivo were disrupted by systemic administration of the TAT-101 peptide. WT mice were injected with either control TAT or TAT-101 (i.p. 50 mg/kg) and hippocampi were collected 2 h later for immunoprecipitation and Western blot. Immunoprecipitation under denaturing conditions was performed with anti-SK2 antibodies or control IgG and Western blots were labeled with anti-ubiquitin antibodies (right panel). Arrows indicate ubiquitinated SK2 species. Levels of input proteins were also evaluated by Western blot probed with SK2 and β-actin antibodies (left panel). See also Figure S3.