Abstract
Background
As the pituitary gland develops, signals from the hypothalamus are necessary for pituitary induction and expansion. Little is known about the control of cues that regulate early signaling between the two structures. Ligands and receptors of the Notch signaling pathway are found in both the hypothalamus and Rathke’s pouch. The downstream Notch effector gene Hes1 is required for proper pituitary formation, however these effects could be due to the action of Hes1 in the hypothalamus, Rathke’s pouch or both. To determine the contribution of hypothalamic Notch signaling to pituitary organogenesis, we used mice with loss and gain of Notch function within the developing hypothalamus.
Results
We demonstrate that loss of Notch signaling by conditional deletion of Rbpj in the hypothalamus does not affect expression of Hes1 within the posterior hypothalamus or expression of Hes5. In contrast, expression of activated Notch within the hypothalamus results in ectopic Hes5 expression and increased Hes1 expression, which is sufficient to disrupt pituitary development and postnatal expansion.
Conclusions
Taken together, our results indicate that Rbpjdependent Notch signaling within the developing hypothalamus is not necessary for pituitary development, but persistent Notch signaling and ectopic Hes5 expression in hypothalamic progenitors affects pituitary induction and expansion.
Keywords: pituitary development, Notch signaling, Hes1, Hes5, infundibulum
INTRODUCTION
Rathke’s pouch (RP), the primordium of the anterior lobe (AL) and intermediate lobe (IL) of the pituitary, arises from a distinct region of oral ectoderm midline, which lies in contact with the adjacent hypothalamus. As development continues, the infundibulum (INF), a portion of the contacting hypothalamus, envaginates into RP to form the posterior lobe (PL) of the pituitary (Schwind, J.L. 1928; Kaufman, 1992). The notion that hypothalamic signaling influences pituitary development has been well characterized by tissue recombination studies demonstrating that the hypothalamus is critical for appropriate pituitary expansion (Ericson et al., 1998, Norlin et al., 2000). However, molecular mechanisms within the hypothalamus that direct pituitary formation are not well understood.
Known signals exchanged between the developing pituitary and hypothalamus include factors such as SHH, BMP, FGF and Wnt proteins (Dale et al., 1997, Manning et al., 2006), derived from both the primordial endocrine hypothalamus (BMP4, FGF8, FGF10, Wnt5a, SHH), and the oral ectoderm (SHH, BMP2, Wnt4; Alatzoglou and Dattani, 2009, Zhao et al., 2012). These signaling molecules initiate expression of genes within RP, such as Lhx3, Lhx4 and Isl1, which control the initial steps of pituitary organogenesis (Treier and Rosenfeld, 1996, Takuma et al., 1998, Ericson et al., 1998, Norlin et al., 2000, Rosenfeld et al., 2000, Zhu et al., 2007). Despite their importance in controlling pituitary organogenesis, there is limited information regarding the developmental pathways that regulate expression of hypothalamic morphogens such as BMP and FGF. One important transcription factor is Nkx2.1, which is expressed within the hypothalamus and restricted from RP (Lazzaro et al., 1991). Nkx2.1 is required for FGF8 expression in the infundibulum (Takuma et al., 1998) and targeted deletion of Nkx2.1 results in early developmental arrest of pituitary organogenesis just after RP formation (Kimura et al., 1996, Takuma et al., 1998). These studies indicate that the Nkx2.1 expressing hypothalamic progenitors are necessary for pituitary development.
Once RP is induced with assistance from hypothalamic cues, proopriomelanocortin (POMC)-positive corticotropes that produce adrenocorticotropic hormone (ACTH) are the first cells to differentiate within RP of mice at embryonic day 12.5 (e12.5). Corticotrope differentiation is followed by differentiation of thyroid stimulating hormone (TSH)-positive thyrotropes at e14.5, growth hormone (GH)-positive somatotropes at e15.5, prolactin (PRL)-positive lactotropes at e16.5, and finally follicle stimulating hormone (FSH) and luteinizing hormone (LH) expressing gonadotropes around e16.5 (Simmons et al., 1990, Japon et al., 1994). Melanotropes, found only in the IL and detected by POMC expression, begin producing melanocyte stimulating hormone (MSH) at e16.5 (Davis et al., 2011).
Members of the Notch signaling pathway are expressed in both the developing pituitary and hypothalamus (Raetzman et al., 2004) and have shown to be important for RP development and hormone specification (Ward et al., 2005, Raetzman et al., 2006, Vesper et al., 2006, Raetzman et al., 2007, Himes and Raetzman, 2009, Monahan et al., 2009). Notch receptors and ligands are transmembrane proteins that allow cell-to-cell signaling between adjacent cells. Canonical Notch ligand activation results in cleavage of the Notch intracellular domain (NICD), which then translocates to the nucleus and associates with the Rbpj/Mastermind (MAM) complex (Selkoe and Kopan, 2003). The NICD/Rbpj/MAM complex then induces transcription of basic helix-loop-helix (bHLH) transcription factors, such as Hes and Hey genes (Iso et al., 2003). However, studies have shown that through Notch-independent pathways, Hes genes can be expressed in the absence of Rbpj (Martinez Arias et al., 2002, Brennan and Gardner, 2002). Additionally, Rbpj/MAM can form transcriptional complexes that function independently of Notch signaling, including repression and activation of target genes (Johnson and Macdonald, 2011).
The Notch target Hes1 has been shown to be important for development of both the hypothalamus and pituitary (Aujla et al., 2011, Raetzman et al., 2007, Kita et al., 2007, Aujla et al., 2013). More specifically, global Hes1 loss results in ectopic expression of Hes5, decreased cell proliferation and increased cell death within pituitary progenitor cells, coincident with loss of LHX3 (Raetzman et al., 2007, Kita et al., 2007). Hes1 expression is also necessary for proper melanotrope specification within the pituitary, possibly by restricting the expression of the transcription factor PIT1 (Raetzman et al., 2007). Additional studies have also used a conditional deletion approach to define the role of Notch signaling specifically in pituitary progenitors. Pituitary specific loss of Rbpj results in hypopituitarism with absence of the somatotrope, thyrotrope and lactotrope lineages (Zhu et al., 2006). The phenotype observed by pituitary specific loss of Rbpj-dependent Notch signaling is less severe than loss of Hes1 in both the pituitary and hypothalamus, suggesting that Notch signaling within the hypothalamus or cross-talk between the hypothalamus and pituitary is important for pituitary development. However, although the role of Notch signaling during pituitary development and cell specification has been explored, relatively little is known regarding the role of Notch within the hypothalamus itself and how this may impact RP development.
To determine if Notch signaling within the developing hypothalamus influences pituitary formation, we analyzed pituitary development in mice with hypothalamic-specific loss and gain of Notch function. We demonstrate that loss of Rbpj-dependent Notch signaling in the hypothalamus selectively eliminates Hes1 from the anterior hypothalamus (Aujla et al., 2013). However, loss of Notch/Rbpj signaling does not eliminate Hes1 from the posterior hypothalamus and has no effect on Hes5 expression or pituitary formation. In contrast, persistent activation of Notch within the hypothalamus is sufficient to cause loss of the IL and PL, which are still absent at 8 postnatal weeks. Additionally, we show that persistent hypothalamic Notch activation results in a reduction of OTX2 and Fgf10 expression within the infundibulum, coincident with a reduction in LHX3 expression and aberrant cell death in RP. Persistent Notch expression additionally affects the boundaries of Shh expression within the posterior hypothalamus. Interestingly, persistent Notch expression within the hypothalamus does not affect specification of AL hormone cells, but does influence shape of the developing pituitary, and pituitary size is significantly reduced at 8 postnatal weeks. Taken together, we demonstrate that persistent Notch expression affects structural formation of the PL, morphology and proper cell specification within the IL, but not hormonal cell specification within the developing AL. Additionally, our results indicate that ectopic expression of Hes5 resulting from either persistent Notch expression or Hes1 loss have significant consequences on pituitary development.
RESULTS
Persistent expression of Notch and loss of Hes1 both result in ectopic Hes5 expression while loss of Rbpj does not alter Hes1 or Hes5 within the posterior hypothalamus
Notch signaling is active in the developing hypothalamus and pituitary and loss of function in both structures affects formation of the PL as well as differentiation and size of the IL and AL (Raetzman et al., 2004, Ward et al., 2005, Raetzman et al., 2006, Vesper et al., 2006, Raetzman et al., 2007, Kita et al., 2007, Monahan et al., 2009, Himes and Raetzman, 2009, Akimoto et al., 2010). To determine the role of hypothalamic Rbpj-dependent Notch signaling in pituitary organogenesis, we employed conditional deletion of an essential cofactor of Notch receptors, Rbpj, in the developing hypothalamic progenitors (Han et al., 2002). Rbpjfl/fl mice (control) were bred to Nkx2.1-CRE mice (Lazzaro et al., 1991, Xu et al., 2008) to generate Rbpjfl/fl Nkx2.1-CRE (Rbpj cKO) mice. To determine the effects of Notch overexpression within the developing hypothalamus, we utilized a tissue specific approach employing mice that express one copy of the constitutively active Notch1 intracellular domain (NICD) within the hypothalamus (Murtaugh et al., 2003). RosaNotchICD/+ mice (control) were bred to Nkx2.1-Cre mice, generating RosaNotchICD/+ Nkx2.1-Cre (NICD Tg) mice with persistent Notch activation in the Nkx2.1-positive hypothalamic progenitors by e9.5 (Shimogori et al., 2010, Ring and Zeltser, 2010, Ferri et al., 2013).
We examined expression of the Notch target Hes5 in order to examine how loss of Rbpj-dependent Notch signaling or persistent Notch activation would affect its expression at e10.5. We found that expression of Hes5 is normally restricted to the anterior hypothalamus (Fig. 1A), and that loss of Rbpj-dependent Notch signaling does not affect Hes5 expression within the anterior hypothalamus (Fig. 1B). Additionally, persistent expression of Notch results in ectopic expression of Hes5 within the posterior hypothalamus (Fig. 1C). We found that pituitary morphology in NICD Tg mice resembled that of Hes1 null mice so we examined Hes5 expression within Hes1 null mice and found ectopic Hes5 expression within the posterior hypothalamus and within RP (Fig. 1D). We additionally examined expression of the Notch target Hes1, which is expressed throughout the posterior and anterior hypothalamus in control animals (Fig. 1E). Surprisingly, we found that Hes1 is still present within the posterior hypothalamus in Rbpj cKO mice, but is absent from the anterior hypothalamus (Fig. 1F). In contrast, persistent expression of Notch appears to increase Hes1 within the anterior and posterior hypothalamus (Fig. 1G). When examining RP, Hes1 expression appears reduced, especially on the caudal side of the NICD Tg pituitary (Fig. 1G, inset), compared to the control (Fig. 1E, inset).
Figure 1. Loss of Rbpj does not alter Hes1 or Hes5 expression within the posterior hypothalamus, while both persistent Notch activation and Hes1 loss results in ectopic Hes5 within the posterior hypothalamus.
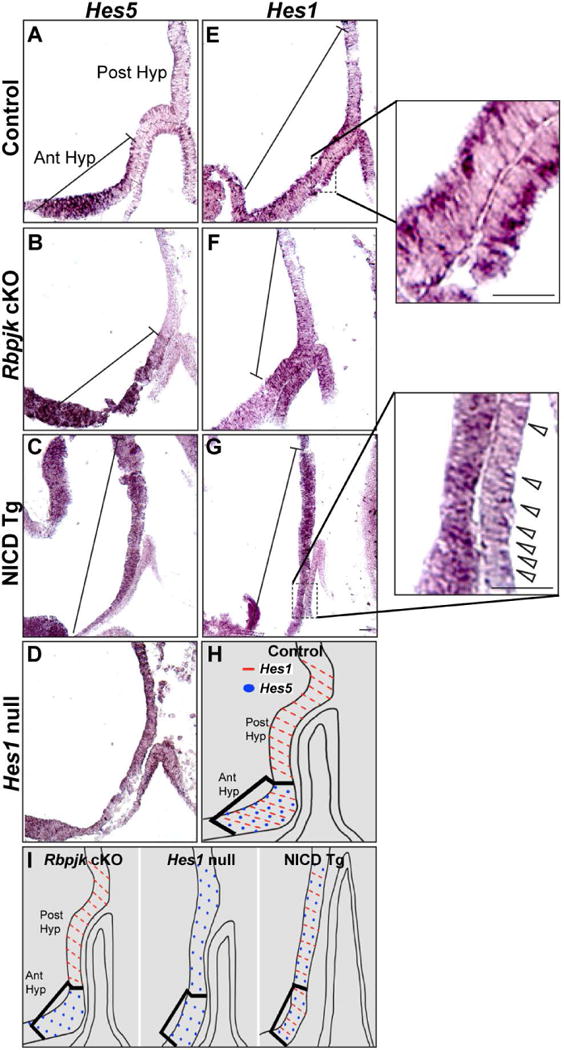
In situ hybridization performed on sagittal sections at e10.5 shows Hes5 expression restricted within the anterior hypothalamus in control (A) and RbpjcKO (B) mice. In contrast, NICD Tg mice (C) and Hes1 null mice (D) have ectopic Hes5 expression within the posterior hypothalamus. Additionally, Hes1 is expressed within the anterior and posterior hypothalamus in control mice (E), is absent from the anterior hypothalamus in RbpjcKO mice (F) and appears increased within both the anterior and posterior hypothalamus in NICD Tg mice (G). Hes1 expression appears reduced in Rathke’s Pouch, especially on the caudal side of the NICD Tg pituitary (Fig. 1G, inset), compared to control (Fig. 1E, inset). H, I: A schematic summarizing Hes1 (red dash) and Hes5 (blue dot) expression within the anterior and posterior hypothalamus in control, RbpjcKO,Hes1 null and NICD Tg mice. Scale bar, 50μm.
In summary, in control animals Hes5 is restricted to the anterior hypothalamus, while Hes1 is expressed in both regions (Fig. 1H). Loss of Rbpj-dependent Notch signaling results in loss of Hes1 only from the anterior hypothalamus and does not affect Hes5 expression, while both Hes1 null and NICD Tg have ectopic Hes5 expression throughout the posterior hypothalamus (Fig. 1I). Taken together, our results indicate that Hes genes may be regulated independently of Rbpj. Given that both Hes1 null mice and NICD Tg mice exhibit similar pituitary phenotypes, we further characterized how persistent Notch expression may affect RP formation.
Persistent activation of hypothalamic Notch affects expression of patterning factors within the hypothalamus that communicate with Rathke’s pouch
In control mice at e10.5, RP has separated from the oral ectoderm and the infundibulum (INF) has formed by evagination of the neural ectoderm (Fig 2A). Persistent activation of NICD within the developing hypothalamus allows formation of RP at e10.5, but the structure appears thinner and elongated with no apparent INF formation (Fig 2B). To investigate how persistent expression of Notch may affect expression of hypothalamic patterning genes crucial for proper pituitary formation, we examined the expression of Shh, OTX2 and Fgf10. Proper restriction of Shh is crucial to pituitary development (Zhao et al., 2012). We found that expression of Shh, which is restricted to the anterior hypothalamus (brackets) of control mice (Fig. 2C), has altered expression boundaries in NICD Tg mice (brackets, Fig. 2D).
Figure 2. Persistent activation of hypothalamic Notch affects expression of patterning factors within the hypothalamus and Rathke’s pouch.
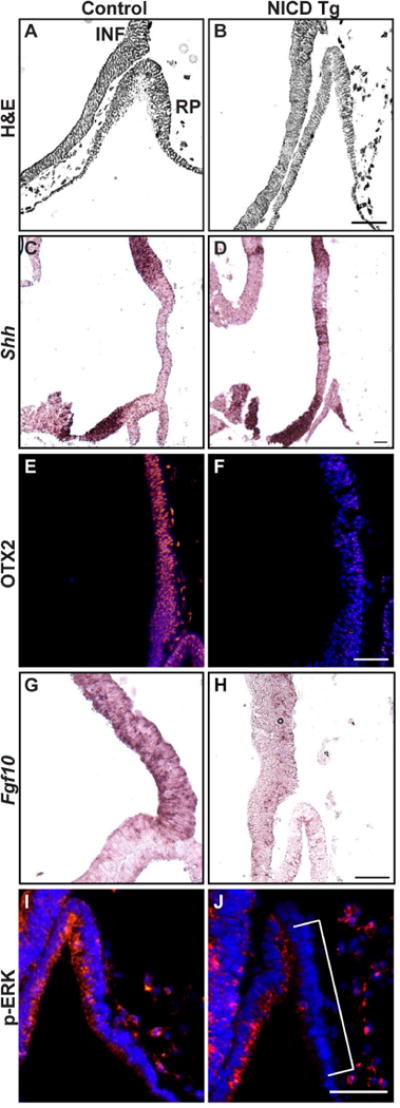
Hematoxylin and eosin (H&E) staining in sagittal sections at e10.5 shows the morphology of control (A) and NICD Tg (B) hypothalamic (Hyp) and pituitary regions, displaying loss of the infundibulum (INF) and thin RP in NICD Tg mice. Expression of Shh mRNA, detected by in-situ hybridization, is restricted to the anterior hypothalamus in control mice (C), while boundaries of Shh expression are disrupted in NICD Tg mice (D). Additionally, persistent expression of Notch1 results in loss of OTX2 expression within the INF (F) compared to controls (E). Another important hypothalamic patterning gene, Fgf10, is normally expressed throughout the INF in control mice (G), and has reduced expression in NICD Tg animals (H). Immunohistochemistry shows that expression of p-ERK, which is uniformly expressed in RP of control animals (I), is lost on the caudal side of RP in Tg animals (brackets, J). Scale bar, 50μm.
To examine posterior hypothalamic patterning, we examined OTX2, which is present within the forming INF and posterior hypothalamic neuroepithelium (Mortensen et al., 2011). We found that OTX2 is expressed within the INF in control animals (brackets, Fig 2E), and is reduced in NICD Tg animals (brackets, Fig 2F). To further investigate INF differentiation and signaling, we examined Fgf10 mRNA, which is restricted to the INF of control animals (Fig 2G). Fgf10 expression is dramatically reduced in the hypothalamus of NICD Tg animals (Fig 2H). Immunohistochemistry reveals uniform expression of phosophorylated-ERK1/2 (pERK) within RP, indicating activation of FGF signaling within the control pituitary (Powers et al., 2000, Corson et al., 2003) Fig 2I). In contrast, NICD Tg animals display p-ERK immunoreactivity restricted to the rostral side of RP (Fig 2J), which potentially indicates restricted activation of FGF signaling within the pituitary from the hypothalamus. Overall, these data suggest that persistent expression of Notch signaling in the hypothalamus alters INF formation as well as OTX2, FGF, and Shh expression, which may affect signaling from the hypothalamus to the pituitary and alter pituitary development.
NICD activation within the developing hypothalamus affects patterning and survival of pituitary progenitors
Cells within RP depend on FGF signaling from the posterior hypothalamus in order to induce intrinsic pituitary factors such as the LIM homeodomain transcription factor LHX3 (Sheng et al., 1996, Ericson et al., 1998, Treier et al., 1998, Norlin et al., 2000, Charles et al., 2005). LHX3 is normally expressed throughout RP at e10.5 (Fig. 3A), but this expression is reduced in NICD Tg mice and restricted from the caudal side and dorsal tip of RP (Fig. 3B). An additional LIM homeodomain transcription factor, ISL1, is the first LIM protein to be expressed during pituitary development and becomes restricted to the ventral portion of the pouch by e10.5 (Zhu et al., 2007; Fig 3C). In contrast, ISL1 immunoreactive cells in NICD Tg pituitaries are not ventrally restricted and are also present in the dorsal region of the pouch (Fig 3D). Consistent with LHX3 expression at e10.5, visualization by TUNEL immunohistochemistry illustrates that there are no dying cells within Rathke’s pouch (Fig. 3E, Raetzman et al., 2007, Monahan et al., 2009), while NICD Tg mice have apoptotic cells in caudal side of RP where LHX3 expression is eliminated (Fig. 3F).
Figure 3. Persistent hypothalamic Notch expression affects survival of pituitary progenitors at e10.5.
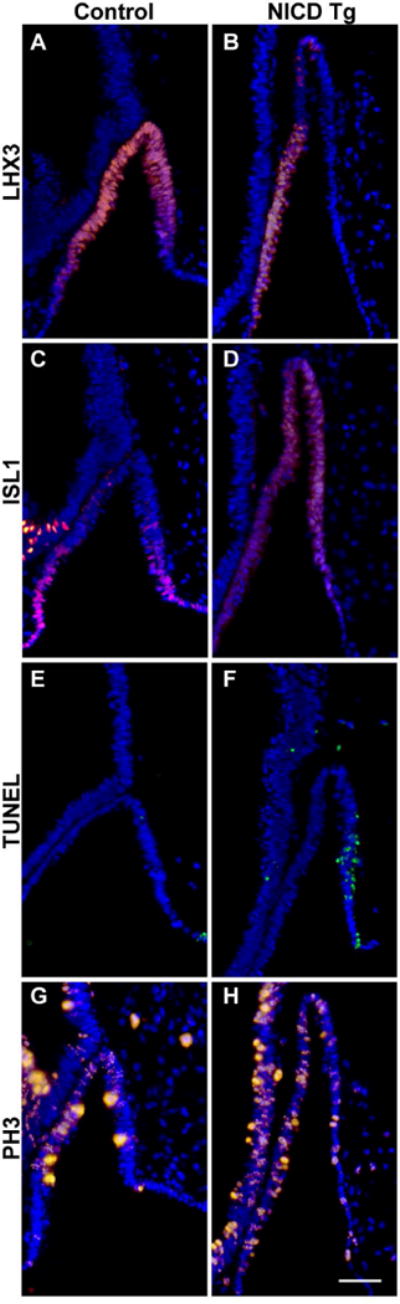
Control sagittal sections at e10.5 show LHX3 immunopositive cells within Rathke’s pouch (RP), allowing for proper pituitary induction (A). NICD Tg animals display reduced LHX3 expression, specifically on the caudal and dorsal aspect of RP (B). ISL1 immunopositive cells, restricted to the ventral aspect of RP in control animals (C), are instead expressed uniformly in NICD Tg animals (D). TUNEL-reactive cells, representing cells undergoing cell death, are not present in control pituitaries at e10.5 (E), but are present in the caudal side of RP in Tg animals (F). The percent of immunoreactive phospho-histone-H3 (PH3) cells over total DAPI-postitive cells is significantly reduced in NICD Tg pituitaries (H, 34.7±0.6%, p=0.03) compared to control pituitaries (G, 42.1±1.9%). Scale bar, 50μm.
We examined proliferation of pituitary progenitors within RP by immunohistochemistry labeling phospho-histone H3 (PH3) in order to visualize cells undergoing mitosis. We found that NICD Tg animals (Fig. 3H; 138.6±7.8, p=0.03) have significantly more DAPI-positive cells in the elongated RP compared to controls (Fig. 3G; 96.3±9.8). We then counted PH3-immunopositive cells within RP to determine the average percent of PH3-immunopositive cells per total cells and found that NICD Tg pituitaries have a significantly lower percentage of proliferating cells (Fig. 3H; 34.7±0.6%, p=0.03) compared to control pituitaries (Fig. 3G; 42.1±1.9%, detailed counts in Table 1). In the course of these experiments, we also examined proliferation within the ventral diencephalon. NICD Tg mice do not show any significant difference in percent of PH3-immunopositive cells within the ventral diencephalon (B; 23.5±1.9%; p=0.14) compared to controls (A; 19.7±0.74). However, there did appear to be subtle regional differences in proliferation, especially in the area where the PL arises from in the control embryos, which has subtly less PH3-immunopositive cells compared to NICD Tg mice. Taken together, our results indicate that Notch overexpression within the hypothalamus results in disruption of proper pituitary induction, increased cell death and decreased proliferation.
Table 1.
Average number of PH3-immunpositive cells in RP at e10.5
| Average (± SEM) | NICD Control | NICD Tg | p-value |
|---|---|---|---|
| Number of cells in RP | 96.3±9.8 | 138.6±7.8 | *p=0.025 |
| Total PH3+ cells in RP | 40.6±5.9 | 48.1±2.5 | p=0.343 |
| Percent of PH3+ cells/total cells in RP | 42.1±1.9% | 34.7±0.6% | *p=0.029 |
| Percent of late G2 phase PH3+ cells/total cells in RP | 33.2±2.5% | 30.6±2.5% | p=0.44 |
| Percent of M phase PH3+ cells/total Cells in RP | 8.82±0.58% | 6.51±0.34% | p=0.17 |
Persistent Notch activation in the developing hypothalamus affects pituitary morphology but not hormone specification
To determine if extrinsic Notch signaling from the hypothalamus affects pituitary hormone cell specification, we examined NICD Tg and control pituitaries at e16.5. Overexpression of NICD results in loss of a defined PL and IL (Fig. 4B anterior, Fig 4D posterior) compared to controls (Fig. 4A, anterior, Fig. 4C posterior), although a small cleft can be observed in NICD Tg mice (Fig. 4D, posterior, bracket). Hormone cells such as LH (Fig. 4E), GH (Fig. 4G), TSH (Fig. 4I) and POMC derivatives (Fig. 4K) are normally specified in AL by e16.5. NICD Tg animals display similar specification of these hormone cell types within the AL at the same age, though the structure itself is elongated into the forming sphenoid bone (Fig. 4F–4L). Our results indicate that persistent Notch signaling within the hypothalamus does not affect terminal differentiation of pituitary hormone producing cells within the AL, but does affect pituitary shape.
Figure 4. Persistent hypothalamic Notch activation disrupts pituitary morphology but not hormone specification at e16.5.
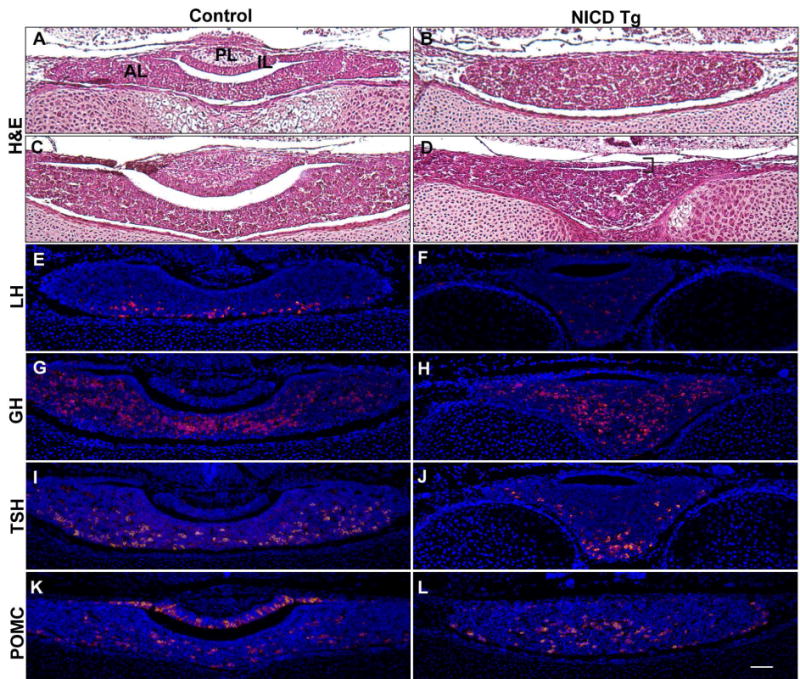
Hematoxylin and eosin (H&E) staining reveals proper anterior lobe (AL), intermediate lobe (IL) and posterior lobe (PL) morphology in coronal pituitary sections at e16.5 (A, anterior; C, posterior). In contrast, NICD Tg pituitaries have altered pituitary shape with lack of IL distinction and no PL formation (B, anterior; D, posterior). In control pituitaries, luteinizing hormone (LH; E), growth hormone (GH; G), thyroid-stimulating hormone (TSH; I), proopiomelanocortin (POMC; K) are specified by e16.5. NICD Tg pituitaries also display specification of these hormones (F, H, J, L). Scale bar, 50m.
NICD activation affects proper intermediate lobe formation and intermediate lobe hormone expression
In order to more carefully examine the dysmorphic region dorsal to the cleft in NICD Tg pituitaries at e16.5, we used immunohistochemistry to determine if this region contained intermediate lobe cell types. We found that Sox2, a marker of pituitary progenitors (Fauquier et al., 2008), is present in cells within the IL of control animals at e16.5 (Fig. 5A), and is also present surrounding the cleft of NICD Tg pituitaries (Fig. 5B). We then examined PAX7, a marker of melantropes within the IL (Hosoyama et al., 2010, Budry et al., 2012), which is robustly expressed within the IL of control mice at e16.5 (Fig. 5C). NICD Tg pituitaries display no PAX7 immunopositive cells in the thin cell layer above the cleft (Fig. 5D) or within rest of the pituitary (data not shown), indicating that the IL may be not properly formed in these animals. Cells containing POMC derivatives melanocyte stimulating hormone (MSH) found in the IL, and adrenocorticotropic (ACTH) found in the AL, are both detected by POMC antibody in control animals at e16.5 (Fig. 5K, Fig. 5E). In contrast, immunoreactive POMC cells are reduced in the region superior to the cleft of NICD Tg animals (Fig. 5F). Additionally, the morphology of POMC-immunopositive cells found in the region above the cleft in NICD Tg pituitaries resembles that of ACTH-positive cells found in the AL of controls, indicating that this region of cells may be an extension of AL surrounding the cleft.
Figure 5. Activated Notch in the developing hypothalamus affects intermediate lobe (IL) specification at e16.5.
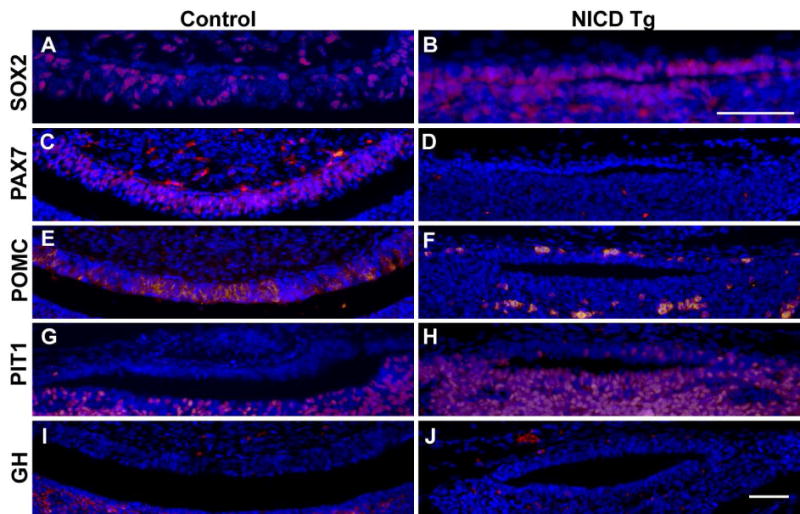
Coronal sections display Sox2 immunopositive cells in the IL of control animals (A), and Sox2 immunopositive cells are also present in the region surrounding the cleft in NICD Tg pituitaries (B). The IL specific marker, PAX7, is present throughout the IL of control pituitaries (C), but is absent in NICD Tg pituitaries (D). POMC derivatives are found in the IL of control animals (E) and immunoreactive POMC cells are reduced in the region above the cleft in NICD Tg animals (F). Control animals show restriction of PIT1 from the IL (G), while NICD Tg pituitaries display PIT1 expression within the region superior to the cleft (H). Additionally, growth hormone (GH) cells, a lineage requiring PIT1 expression, are restricted from the IL in control pituitaries (I) and also from the region superior to the cleft in NICD Tg pituitaries (J). Scale bar, 50μm.
In order to determine what cell types are present in the region superior to the cleft within NICD Tg animals, pituitaries were immunostained with an antibody to the homeodomain transcription factor PIT1. Expression of PIT1, normally localized to the AL, is required for TSH, GH and prolactin (PRL) expression as well as proliferation of thyrotropes, somatotropes and lactotropes (Lin et al., 1994, Ward et al., 2006, Zhu et al., 2006). In control animals, PIT1 is restricted from the IL (Fig. 5G), but PIT1 immunopositive cells are found in the region above the cleft in NICD Tg animals (Fig. 5H), indicating that there is no IL delineation. Interestingly, GH expression, which requires PIT1, is restricted from both the IL in control animals (Fig. 5I), as well as the cells above the cleft in NICD Tg animals (Fig. 5J). These data show that the cell layer above the cleft found in NICD Tg pituitaries does not differentiate into the IL, though Sox2 containing cells still form the boundary of the cleft. Taken together, these results indicate that persistent expression of Notch affects the boundary and formation of the IL and the AL within the developing pituitary.
Persistent activation of NICD results in a smaller pituitary with no PL in adulthood
The pituitary substantially increases in size postnatally with clear delineation of AL, PL and IL in gross structure (Fig. 6A) and in histological sections throughout the pituitary (Fig. 6B). NICD Tg animals have a severely affected pituitary shape and significantly reduced size (Fig. 6B, 6D). NICD Tg pituitaries are further set within the hypophyseal fossa of the sphenoid bone compared to controls (data not shown), and maintain the elongated shape observed in sagittal sections at e10.5 (Fig. 6B, Fig. 6D). Throughout sections of the NICD Tg pituitary, no distinct IL or PL morphological regions are found. Additionally, a region of tissue 500μm from the AL on a transverse section, yet still connected to the main AL tissue, was present (Fig. 6D).
Figure 6. Persistent activation of hypothalamic Notch results in maintained abnormal pituitary morphology, reduced pituitary size but does not affect hormone specification at 8 postnatal weeks.
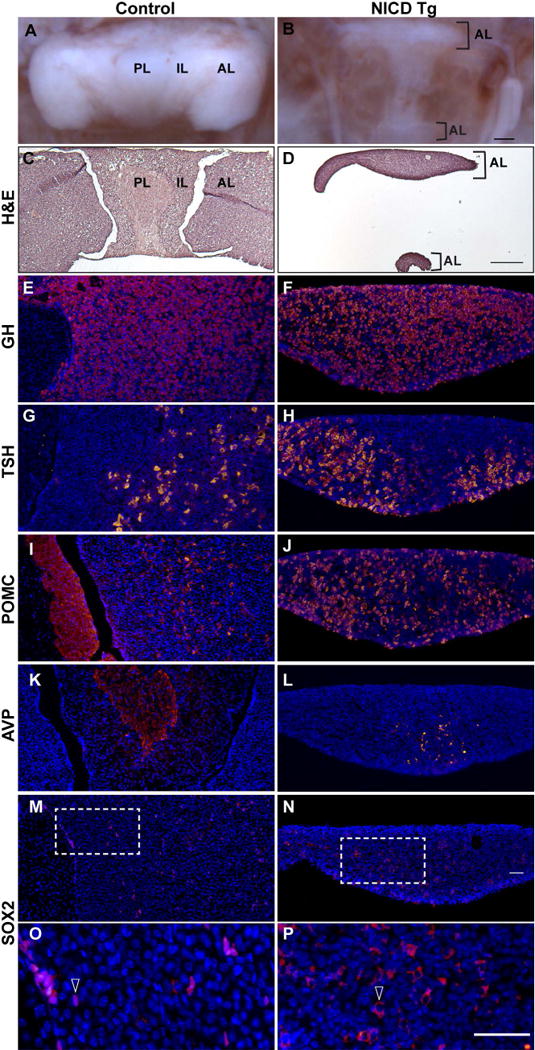
Pituitary photographs reveal the anterior lobe (AL), intermediate lobe (IL), and posterior lobe (PL; A). NICD Tg mice show dramatic reduction in pituitary size, as well as elongated pituitary shape and a lack of distinct IL and PL (B). Hematoxylin and eosin (H&E) staining shows morphology of control (C) and NICD Tg (D) pituitaries. In control pituitaries, growth hormone (GH; E), thyroid stimulating hormone (TSH; G) and proopiomelanocortin (POMC, I) are specified appropriately in the AL and IL. NICD Tg animals show specification of these hormones in the AL, but lack a region that resembles an IL or PL (F, H, J). Arginine vasopressin (AVP), found exclusively in the PL of control animals (K), is detected in the presumed AL of NICD Tg pituitaries (L). Sox2 immunopositive cells are present in the AL and IL of control animals (M) and within the AL of NICD Tg pituitaries (N). Higher magnification reveals cytoplasmic Sox2 immunolocalization in NICD Tg pituitaries (P, arrow), compared to immunostaining localized to the nucleus in controls (O, arrow). Scale bar, 250μm (A–D), 50μm (E–P).
At 8 postnatal weeks, the pituitary displays robust levels of GH within the AL in control (Fig. 6E) and NICD Tg (Fig. 6F) animals. TSH is also detected in both control (Fig. 6G) and NICD Tg pituitaries (Fig. 6H). Both MSH within the IL and ACTH in the AL are detected with POMC antibody in control pituitaries (Fig. 6I), and POMC immunopositive cells are also found in NICD Tg pituitaries (Fig. 6J). Interestingly, while arginine vasopressin (AVP), staining axonal processes from hypothalamic magnocellular neurons, is appropriately restricted to the PL of control pituitaries (Fig. 6K), AVP-immunopositive processes are detected in the medial aspect of the AL in NICD Tg pituitaries (Fig. 6L). The ectopic pituitary tissue shown in Fig. 6D contained AL hormones such as GH, TSH, POMC, but did not contain AVP processes (data not shown). Sox2 is present in cells lining the cleft and in isolated AL cells of control pituitaries at 8 weeks (Fig. 6M), as well as in the AL of NICD Tg mice (Fig. 6N). Sox2 expression is maintained in the nucleus within cells in the control (Fig. 6O, arrow), but in contrast cytoplasmic Sox2 immunoreactivity is observed in NICD Tg pituitaries (Fig. 6P, arrow). Taken together, these data suggest that although pituitary hormone expression is not affected by persistent hypothalamic NICD expression the pituitary morphology, pituitary size and Sox2 expression are profoundly disrupted.
Loss of Rbpj in the developing hypothalamus does not affect pituitary hormone cell specification at e16.5
Because the loss of Rbpj affected cell numbers in the developing arcuate nucleus (Aujla, et al. 2013), we examined the effect of this hypothalamic alteration on pituitary hormone expression at e16.5. Loss of Rbpj dependent Notch signaling had no discernable effect on pituitary structure when comparing control (Fig. 7A) to Rbpj cKO (Fig. 7B) mice. LH is not readily detectable at this age in either control (Fig. 7C) or Rbpj cKO pituitaries (Fig. 7D). Hormone cells containing GH (Fig. 7E), TSH (Fig. 7G) and POMC (Fig. 7I) are present in control pituitaries. Rbpj cKO animals display no obvious difference in cell specification when compared to controls (Fig. 7F, H & J). Our results indicate that loss of hypothalamic Rbpj dependent Notch signaling does not overtly affect pituitary development.
Figure 7. Loss of Rbpj dependent hypothalamic Notch signaling does not affect pituitary hormone cells at e16.5.
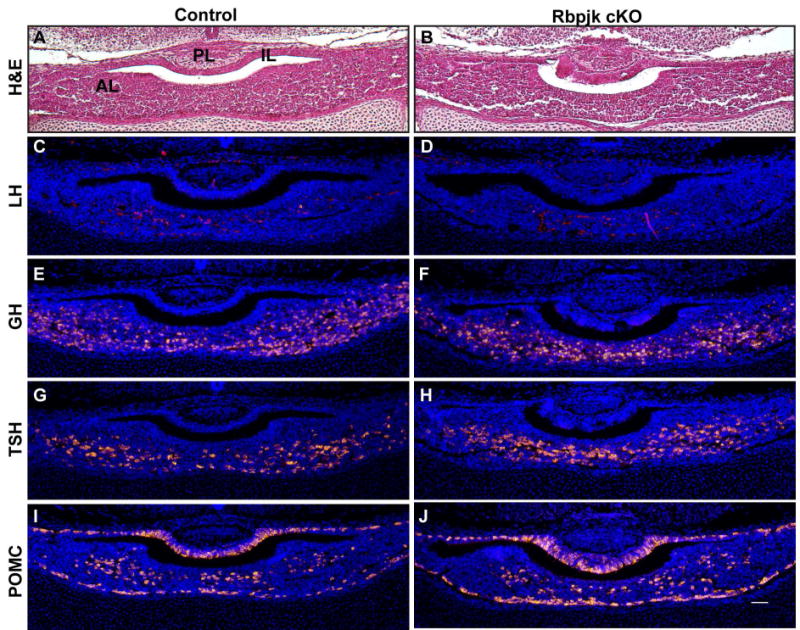
Hematoxylin and eosin (H&E) staining reveals anterior lobe (AL), intermediate lobe (IL) and posterior lobe (PL) morphology in coronal pituitary sections of control (A) and Rbpj cKO (B) mice. Luteinizing hormone (LH) is not readily detectable in either control (Fig. 7C) or Rbpj cKO pituitaries (Fig. 7D) at e16.5. In control pituitaries, growth hormone (GH; E), thyroid stimulating hormone (TSH; G), proopiomelanocortin (POMC; I) are specified by e16.5. RbpjcKO pituitaries also display specification of these hormone producing cells (F, H, J). Scale bar, 50μm.
DISCUSSION
Notch signaling is present within the developing pituitary as well as hypothalamus and loss of the Notch effector gene Hes1 in both tissues results in hypopituitarism, coincident with a reduced PL (Raetzman et al., 2004, Raetzman et al., 2007, Aujla et al., 2011). The hypopituitarism observed with global Hes1 loss could result from Notch signaling reduction in the hypothalamus, pituitary or both structures. In order to address if Notch signaling within the developing hypothalamus specifically is important for pituitary development, we utilized mice with hypothalamic specific loss of Notch/Rbpj signaling and compared them to Hes1 null mice. Our studies reveal that in Hes1 null mice, Hes5 is robustly and ectopically expressed within the posterior hypothalamus and that persistently expressing Notch1 intracellular domain in the anterior and posterior hypothalamus leads to a similar ectopic expression of Hes5 (Fig. 1). The pituitary phenotype observed in NICD Tg mice, including absent INF, increased apoptosis, reduced progenitor proliferation and reduced LHX3-immunopositive cells (Fig. 3) bears striking resemblance to Hes1 null pituitaries (Raetzman et al., 2007, Monahan et al., 2009). Additionally, recent studies have shown that loss of the hypothalamic patterning factor Tbx3 results in ectopic Hes5 expression, complete INF loss and pituitary malformation (Trowe, et al. 2013), supporting the assertion that restriction of Hes5 from the posterior hypothalamus is a crucial common factor that regulates INF formation and proper RP induction.
In contrast, Hes1 expression appears to be Rbpj-dependent within the anterior hypothalamus (Fig. 1) and Rbpj-independent within the posterior hypothalamus, as loss of Rbpj along the ventral midline selectively eliminated Hes1 from the anterior but not the posterior hypothalamus. Expression of Notch1 and Notch2 receptors are restricted to the anterior hypothalamus at e13.5 (Aujla et al., 2013), indicating that Hes1 expression within the posterior hypothalamus is acting through Rbpj-independent signaling. In fact, expression of Hes1 and other downstream Notch factors can be controlled by mediators of the BMP (Dahlqvist et al., 2003, Itoh et al., 2004) and WNT/β-CATENIN (Axelrod et al., 1996, Hayward et al., 2005) pathways. These pathways are both present within the posterior hypothalamus and could be regulating Hes1 expression in an Rbpj-independent manner. Future studies should selectively eliminate Hes1 from the posterior hypothalamus to determine if Notch signaling within this region is necessary for pituitary development.
An important finding from the current study is that posterior hypothalamic patterning relies on appropriate restriction of Notch signaling. We demonstrate that persistent Notch expression in Nkx2.1-positive cells beginning at e9.5 (Shimogori et al., 2010, Ring and Zeltser, 2010, Ferri et al., 2013) affects the restriction of Shh expression (Fig. 2). Recent studies have shown that Shh restriction from the posterior hypothalamus is a critical factor in INF formation (Trowe et al., 2013). There is precedence for Notch and SHH interaction, as Notch and SHH pathways cooperate to form appropriate progenitor boundaries during neocortical development (Dave et al., 2011). There is also evidence of direct cross-talk between SHH and the downstream Notch effector Hes1 in vitro (Ingram et al., 2008). Therefore, the lack of Shh restriction within the posterior hypothalamus observed in the current study could be due to direct interaction of persistently expressed Hes genes found in NICD Tg mice and SHH signaling factors (Fig. 1). Additionally, defects in Shh restriction lead to subsequent reduction in RP proliferation and increased apoptosis (Trowe et al., 2013), similar to phenotype observed in NICD Tg mice. Taken together, these data suggest that persistent expression of Hes1 and Hes5 prevents Shh restriction from the posterior hypothalamus and may contribute to the pituitary malformation observed.
The developing INF represents an important hypothalamic boundary crucial to pituitary development. An important finding in our study is that persistent Notch expression results in absence of INF formation, with a corresponding reduction in OTX2 expression (Fig. 2). Notably, persistent Notch expression within the hypothalamus of NICD Tg mice results in persistent expression of the downstream Notch targets Hes1 and Hes5, and Hes genes have been shown to suppress expression of Otx2 by directly binding to the Otx2 enhancer EELPOT (Ogino et al., 2008, Muranishi et al., 2011). The suppression of Otx2 by Hes genes in retinal progenitor cells maintains their progenitor fate and Hes genes are required to turn off to allow retinal progenitors to become photoreceptor precursors (Muranishi et al., 2011). Taken together with our data showing that persistent Notch expression results in a lack of INF formation, it is possible that Hes genes must be turned off to allow for hypothalamic progenitors to adopt an INF cell fate. In fact, NICD Tg mice appear to have increased numbers of hypothalamic progenitors, whose cell fate as progenitors are maintained throughout embryonic development (Aujla et al., 2013).
The significant reduction in Fgf10 expression in NICD Tg mice offers further evidence that persistent Notch expression affects proper cell specification within the posterior hypothalamus. Notch interaction with FGF signaling pathways is not surprising. In both gastric and pancreatic development, FGF and Notch signaling work together to control progenitor behavior (Miralles et al., 2006). During cardiac development, Notch is necessary for FGF expression (High et al., 2009). Additionally, constitutively active Notch1 or Notch2 can prevent FGF release in vitro (Small et al., 2003). Therefore, in the current study, persistent activation of NICD could lead to the decrease in Fgf10 observed in the posterior hypothalamus.
FGF8 and FGF10 expression within the posterior hypothalamus have been shown to be necessary for LHX3 expression and to maintain cellular proliferation within RP (Ohuchi et al., 2000, Kelberman et al., 2009). Additionally, mice carrying a Fgf8 hypomorphic allele exhibit a smaller pituitary, including a reduced PL and altered IL and AL shape similar to those observed in NICD Tg mice (McCabe et al., 2011). Furthermore, loss of Fgf10 results in apoptosis along the caudal aspect of RP (Ohuchi et al., 2000). These data correspond with this dramatic reduction of Fgf10 found in the posterior hypothalamus of NICD Tg mice, and suggest that loss of FGF signaling activation within RP observed through reduced p-ERK immunoreactivity (Fig. 2) could contribute to the cell death, loss of LHX3 and reduced proliferation found in RP (Fig. 3).
Persistent expression of Notch within the hypothalamus additionally results in an apparent loss of proper IL structure (Fig. 5). The thin layer of cells superior to the cleft in NICD Tg animals is not PAX7-immunopositive and contains aberrant PIT1 positive cells normally found in the AL. PAX7 labels IL progenitor populations, and is required for formation of hormone producing cells within the IL (Hosoyama et al., 2010, Budry et al., 2012). Therefore, persistent NICD expression appears to alter the formation of IL structure as well as IL cell population. When Hes1 is lost in both the hypothalamus and pituitary, IL structure appears thinner and PIT1 positive cells are also ectopically present within the IL. Additionally, IL melanotropes are not specified and IL cells adopt an aberrant somatotrope fate (Raetzman et al., 2007). Taken together, both NICD Tg and Hes1 null mice have disrupted IL structure and abnormal cell specification.
Both NICD Tg and Hes1 null mice additionally have disrupted PL structure (Raetzman et al., 2004, Raetzman et al., 2007, Aujla et al., 2011) and there is precedence for loss of PL structure to disrupt IL cell fate. For example, loss of Lhx2 prevents proper INF formation and evagination into RP and the absence of PL structure results in subsequent malformation of IL structure as the pituitary develops (Zhao, et al. 2010). In the case of Lhx2 mutants, the absence of a PL does not allow RP to extend dorsally and therefore alters IL and AL morphology. Taken together with our data, this suggests that proper formation of the PL structure itself may be a crucial mediator of IL formation.
Interestingly, NICD Tg mice examined at 8 postnatal weeks never develop an IL or PL, and show a significant reduction in AL size compared to controls (Fig. 6), indicating that there is little postnatal pituitary expansion. NICD Tg mice additionally show presence of cytoplasmic expression of Sox2, representing cells in later stages of progenitor development, but not yet differentiated into terminal cell fate (Chen et al., 2009, Gremeaux et al., 2012). Despite the reduction in pituitary size and increased cytoplasmic expression of Sox2, hormone cells in the AL appear to be specified properly, indicating that Notch signaling within the hypothalamus may not affect AL hormone specification, and that intrinsic pituitary factors are important for these factors after initial pituitary induction. This would suggest that although the hypothalamic signals are not essential for pituitary cell differentiation, the disrupted hypothalamic milieu of the NICD Tg mice prevents signals necessary for additional postnatal pituitary growth.
Taken together, our results indicate that Rbpj-dependent Notch signaling within the developing hypothalamus is not necessary for pituitary development, but persistent Notch signaling in hypothalamic progenitors mimics the phenotype of Hes1 null mice and affects pituitary induction and expansion. We show that persistent hypothalamic Notch activation disrupts hypothalamic patterning, coincident with decreased pituitary progenitors. This is likely due a combination of reduced hypothalamic FGF signaling as well as a lack of restricted SHH signaling from the posterior hypothalamus. Disruption of the temporal and spatial control of both FGF and SHH within the hypothalamus along the ventral midline is thought to be a causative factor of Septo-optic dysplasia and accompanying hypopituitarism (McCabe et al., 2011, Zhao et al., 2012). Future studies should selectively eliminate Hes1 and Hes5 from the posterior hypothalamus to determine how Notch interacts with these important pathways.
EXPERIMENTAL PROCEDURES
Animals
RosaNotchICD floxed mice (Murtaugh et al., 2003) purchased from Jackson Laboratories (Bar Harbor, ME, USA) and Rbpj floxed mice (Dr. Tasuku Honjo, Han et al., 2002) were bred to Nkx2.1-cre mice (Lazzaro et al., 1991, Xu et al., 2008) purchased from Jackson Laboratories. Hes1 mutant mice were previously generated by replacing the first 3 exons with a neomycin-resistance cassette (Ishibashi, et al. 1994; gift from Dr. Kageyama). Breeding colonies were generated at the University of Illinois at Urbana-Champaign (UIUC) and all animal procedures were approved by the UIUC Institutional Animal Care and Use Committee. Genotyping was performed as described previously (Lazzaro et al., 1991, Han et al., 2002, Murtaugh et al., 2003).
Histology, immunohistochemistry and in situ hybridization
Mice were collected at e10.5, e16.5 and 8 weeks, and fixed in 3.7% formaldehyde solution (Fisher, Pittsburg, PA) in PBS. For paraffin embedding (e10.5 and e16.5), the samples were dehydrated in a graded series of ethanol before being embedded in paraffin and sectioned coronally or sagittally at 6 μm and mounted onto charged slides. For frozen sectioning (8 weeks), pituitaries were placed in 30% sucrose diluted in PBS overnight, embedded in OCT medium, snap frozen at −80°C and sectioned at 10 μm. A hematoxylin and eosin stain was used to observe cell morphology. To prepare paraffin slides for immunofluorescence, the slides were deparaffinized in xylene and rehydrated in ethanol and PBS followed by a 10 min boil in 10 mM citric acid (pH 6) for slides incubated with anti- phospho-histone-H3 (PH3), anti-Sox2, anti-LHX3, anti-PAX7, anti-pERK, anti-OTX2 or anti-PIT1 antibody. The frozen slides were incubated at room temperature for 10 min, fixed in 3.7% formaldehyde for 10 min, rinsed in PBS, and put in hot citrate for 5 min. All slides were then incubated in normal donkey serum [5% (wt/vol)] diluted in immunohistochemistry block, which consists of PBS, BSA (3%), and Triton X-100 (0.5%), followed by overnight incubation at 4°C with a primary antibody diluted in immunohistochemistry block against the desired peptide: PH3 (1:500, Upstate Cell Signaling Solutions, Lake Placid, NY), Sox2 (1:500, Millipore, Billerica, MA), p-ERK 1/2 (1:300, Santa Cruz Biotechnology, Santa Cruz, CA), LHX3 (1:1000, C651.6DbHN Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), PAX7 (1:500, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), POMC (1:300, Dako, Carpinteria, CA), PIT1 (1:800, a gift from Dr. Simon Rhodes), OTX1/2 (1:500, Abcam, Cambridge, MA), LHβ (1:1500, National Hormone and Pituitary Program-NHPP), FSHβ (1:1800, NHPP), GH (1:1000, NHPP), TSHβ (1:1000, NHPP), and AVP (1:1000, Millipore). Cell death was determined by the TUNEL (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling) method using the in situ cell death detection kit (Roche, Indianapolis IN) according to the manufacturer’s protocol. Donkey-derived mouse and rabbit secondary antibodies conjugated to biotin (Jackson ImmunoResearch, West Grove, PA) were diluted to 1:200 and incubated with sections for 1 h. Slides were then incubated with tertiary antibodies, streptavidin conjugated to either cy2 or cy3 fluorophore (Jackson ImmunoResearch) for 1 h. All sides were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Molecular Probes, Grand Island, NY) at 1:1000 (stock 1 mg/ml) and mounted using aqueous fluorescence mounting media.
For in situ hybridization (ISH) embryos were collected at e10.5 and embedded in paraffin as described for immunohistochemistry. Gene expression was detected with an antisense probe for Hes1 (Akazawa et al., 1992), Hes5 (Akazawa et al., 1992), Shh (gift from Dr. Douglas Epstein (Zhao et al., 2012) and Fgf10 (Bellusci et al., 1997) as previously described (Aujla et al., 2013). For all experiments described, 3–5 mice were analyzed per genotype. Samples were then visualized at 200× magnification using a DM 2560 microscope (Leica, Wetzlar, Germany) and images were obtained using Q Capture Pro software (QImaging, Surrey, British Columbia, Canada) and processed using Photoshop software (Adobe, San Jose, CA).
Quantification of PH3 immunopositive and DAPI positive cells
Sagittal sections throughout the primordial pituitary from control and NICD Tg mice at e10.5 were immunostained with PH3 and DAPI as described. Images were taken at 100× magnification. The number of total PH3-positive cells in RP were counted and divided by the total number of RP cells to obtain the proportion of PH3-positive cells. Total RP cell number was determined by counting DAPI-positive cells in four representative sections from each group and measuring pixel density within the counted region to determine the ratio of DAPI-positive cells per pixel. Pixel density of each section throughout the RP was then determined and multiplied by the ratio to obtain a total number of DAPI-positive cells for each section. For all cell counts, at least 10 sections per animal were analyzed and the average number of PH3-positive cells and DAPI-positive cells was compared between four NICD Tg embryos and four littermate controls in each group. The average number of PH3-immunopositive cells over the average number of total cells for each animal was obtained and the mean and standard deviation of these averages was then calculated for each group. These values were tested for statistical significance using two-tailed t-tests in Microsoft Excel.
Acknowledgments
We thank Leah Nantie and Ashley Himes (Raetzman Lab, University of Illinois at Urbana Champaign) for experimental advice and assistance. We also thank Dr. Sally Camper and Amanda Mortensen at the University of Michigan for experimental advice with OTX1/2 immunohistochemistry. We are grateful to Dr. Thomas Garcia in Dr. Marie-Claude Hoffman’s Lab at UIUC for helpful discussions and providing mice. We also acknowledge members of Dr. Milan Bagchi’s Lab for pERK staining advice and the Lab of Dr. Phil Newmark for use of photography equipment, both at UIUC. This research was funded by National Institutes of Health Grants R01DK076647 (L.T.R.) and F30DK091992 (P.K.A.).
Grant sponsors and grant numbers: NIDDK, R01DK076647 (to L.T.R.) and NIDDK, F30DK091992 (to P.K.A.).
References
- Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–21885. [PubMed] [Google Scholar]
- Akimoto M, Nishimaki T, Arai Y, Uchinuma E, Yamauchi H, Kameda Y. Hes1 regulates formations of the hypophyseal pars tuberalis and the hypothalamus. Cell Tissue Res. 2010;340:509–521. doi: 10.1007/s00441-010-0951-2. [DOI] [PubMed] [Google Scholar]
- Alatzoglou KS, Dattani MT. Genetic forms of hypopituitarism and their manifestation in the neonatal period. Early Hum Dev. 2009;85:705–712. doi: 10.1016/j.earlhumdev.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Aujla PK, Bora A, Monahan P, Sweedler JV, Raetzman LT. The notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary. Dev Biol. 2011;353:61–71. doi: 10.1016/j.ydbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between wingless and notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Brennan K, Gardner P. Notching up another pathway. Bioessays. 2002;24:405–410. doi: 10.1002/bies.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budry L, Balsalobre A, Gauthier Y, Khetchoumian K, L’honore A, Vallette S, Brue T, Figarella-Branger D, Meij B, Drouin J. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev. 2012;26:2299–2310. doi: 10.1101/gad.200436.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27:1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–6099. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, Dodd J, Placzek M. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One. 2011;6:e14680. doi: 10.1371/journal.pone.0014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Mortensen AH, Camper SA. Birthdating studies reshape models for pituitary gland cell specification. Dev Biol. 2011;352:215–227. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Favaro R, Beccari L, Bertolini J, Mercurio S, Nieto-Lopez F, Verzeroli C, La Regina F, De Pietri Tonelli D, Ottolenghi S, Bovolenta P, Nicolis SK. Sox2 is required for embryonic development of the ventral telencephalon through the activation of the ventral determinants Nkx2.1 and shh. Development. 2013;140:1250–1261. doi: 10.1242/dev.073411. [DOI] [PubMed] [Google Scholar]
- Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21:801–813. doi: 10.1089/scd.2011.0496. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A. Notch modulates wnt signalling by associating with armadillo/beta-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. Murine Jagged1/notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119:1986–1996. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol. 2009;325:151–161. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T, Nishijo K, Garcia MM, Schaffer BS, Ohshima-Hosoyama S, Prajapati SI, Davis MD, Grant WF, Scheithauer BW, Marks DL, Rubin BP, Keller C. A postnatal Pax7 progenitor gives rise to pituitary adenomas. Genes Cancer. 2010;1:388–402. doi: 10.1177/1947601910370979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic hedgehog regulates Hes1 through a novel mechanism that is independent of canonical notch pathway signalling. Oncogene. 2008;27:1489–1500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: Multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, ten Dijke Pt P. Synergy and antagonism between notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 2004;23:541–551. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Macdonald RJ. Notch-independent functions of CSL. Curr Top Dev Biol. 2011;97:55–74. doi: 10.1016/B978-0-12-385975-4.00009-7. [DOI] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the snell dwarf mouse reveals pit-1-independent and pit-1-dependent origins of the thyrotrope. Development. 1994;120:515–522. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M. Regional morphogenesis in the hypothalamus: A BMP-Tbx2 pathway coordinates fate and proliferation through shh downregulation. Dev Cell. 2006;11:873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A, Zecchini V, Brennan K. CSL-independent notch signalling: A checkpoint in cell fate decisions during development? Curr Opin Genet Dev. 2002;12:524–533. doi: 10.1016/s0959-437x(02)00336-2. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Gaston-Massuet C, Tziaferi V, Gregory LC, Alatzoglou KS, Signore M, Puelles E, Gerrelli D, Farooqi IS, Raza J, Walker J, Kavanaugh SI, Tsai PS, Pitteloud N, Martinez-Barbera JP, Dattani MT. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endocrinol Metab. 2011;96:E1709–18. doi: 10.1210/jc.2011-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386–4394. doi: 10.1210/en.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi Y, Terada K, Inoue T, Katoh K, Tsujii T, Sanuki R, Kurokawa D, Aizawa S, Tamaki Y, Furukawa T. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J Neurosci. 2011;31:16792–16807. doi: 10.1523/JNEUROSCI.3109-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlin S, Nordstrom U, Edlund T. Fibroblast growth factor signaling is required for the proliferation and patterning of progenitor cells in the developing anterior pituitary. Mech Dev. 2000;96:175–182. doi: 10.1016/s0925-4773(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and notch signaling: A mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120:2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MG, Briata P, Dasen J, Gleiberman AS, Kioussi C, Lin C, O’Connell SM, Ryan A, Szeto DP, Treier M. Multistep signaling and transcriptional requirements for pituitary organogenesis in vivo. Recent Prog Horm Res. 2000;55:1–13. discussion 13-4. [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and presenilin: Regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–1007. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW. Pituitary cell phenotypes involve cell-specific pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4:695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Soldi R, Mandinova A, Kolev V, Trifonova R, Bagala C, Kacer D, Battelli C, Liaw L, Prudovsky I, Maciag T. Notch activation suppresses fibroblast growth factor-dependent cellular transformation. J Biol Chem. 2003;278:16405–16413. doi: 10.1074/jbc.M300464200. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Rosenfeld MG. The hypothalamic-pituitary axis: Co-development of two organs. Curr Opin Cell Biol. 1996;8:833–843. doi: 10.1016/s0955-0674(96)80085-8. [DOI] [PubMed] [Google Scholar]
- Vesper AH, Raetzman LT, Camper SA. Role of prophet of Pit1 (PROP1) in gonadotrope differentiation and puberty. Endocrinology. 2006;147:1654–1663. doi: 10.1210/en.2005-1080. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zevallos SE, Rizzoti K, Jeong Y, Lovell-Badge R, Epstein DJ. Disruption of SoxB1-dependent sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Dev Cell. 2012;22:585–596. doi: 10.1016/j.devcel.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]


