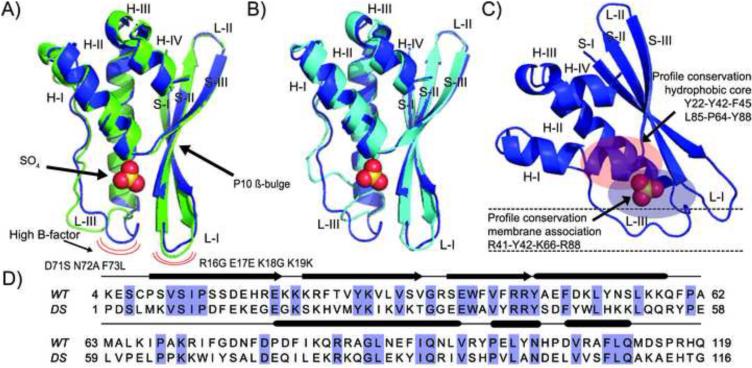
Figure 1.
Structural and sequence comparison between DS-CISK-PX X-ray structure, WT-CISK-PX X-ray structure and DS-CISK-PX I-TASSER model. (A) Superposition of WT-CISK-PX X-ray structure (1XTE) (green) and DS-CISK-PX X-ray structure (blue). Sulfate from DS-CISK-PX shown in spheres to highlight binding cleft. Major structural differences in loop regions having a high B-factor are marked with the red curves. (B) Superposition of DS-CISK-PX X-ray structure (blue) and the I-TASSER DS-CISK-PX model (cyan). C) Predicted orientation of the PX domain relative to the cell membrane (black dash lines) to bind phosphoinositide. D) Sequence alignment between WT-CISK-PX and DS-CISK-PX. Identical residues are highlighted in blue. Secondary structure elements features shared between the two sequences are annotated (strands-arrows, helices-rods, and coils-thin lines).