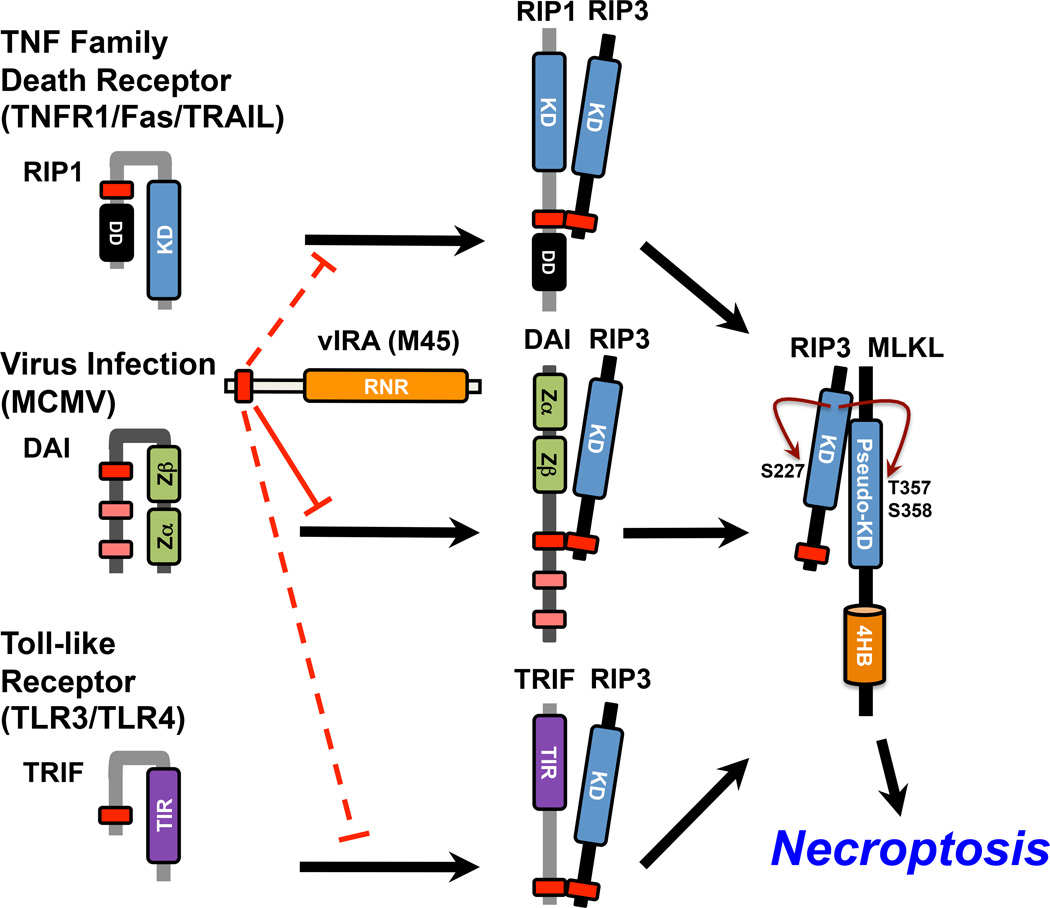
Figure 3. RIP3-mediated necroptosis is activated by three distinct RHIM-containing adaptors and blocked by R1 proteins of MCMV and HSV.
RIP1 is a key pro-necrotic kinase acting downstream of TNF family death receptor (TNFR1, Fas, TRAIL), forming a RIP1–RIP3 complex. Virus-induced DAI–RIP3 necrosis is characterized by MCMV M45 mutant virus infection. In addition to RIP1 and DAI, TRIF, the key TLR3- and TLR4-signaling adaptor, activates RIP3 via TRIF–RIP3 interaction. RIP3 is specifically activated by RIP1, DAI, or TRIF in a RHIM-dependent manner, when it autophosphorylates at S277 and targets MLKL via phosphorylation at T357 and S358. The RHIM competitor MCMV M45 functions during infection to prevent RIP3 association with DAI (solid line), but experimentally can also inhibit association with RIP1 or TRIF (dashed lines).