FIGURE 1.
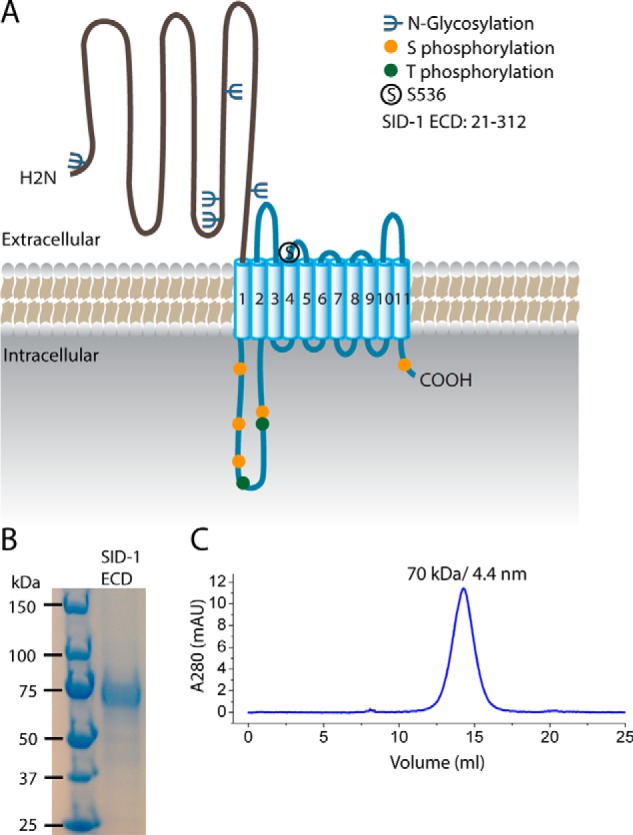
Purification of the SID-1 extracellular domain. A, predicted structural organization and post-translational modifications of SID-1. SID-1 has an extracellular domain (∼300 amino acids) with multiple N-linked glycosylation sites, a multipass transmembrane region carrying a key residue, Ser-536, a cytoplasmic loop having several Ser/Thr phosphorylation sites, and a short cytosolic tail. B, SDS-PAGE analysis and Coomassie Blue staining of purified recombinant SID-1 ECD with hGH-His tag. The disperse band is most likely due to N-linked glycosylation. C, SEC profile of SID-1 ECD using a Superdex 200 column. The main peak corresponds to 70 kDa, as determined by molecular weight standards, and its calculated Stokes radius is 4.4 nm.
