FIGURE 5.
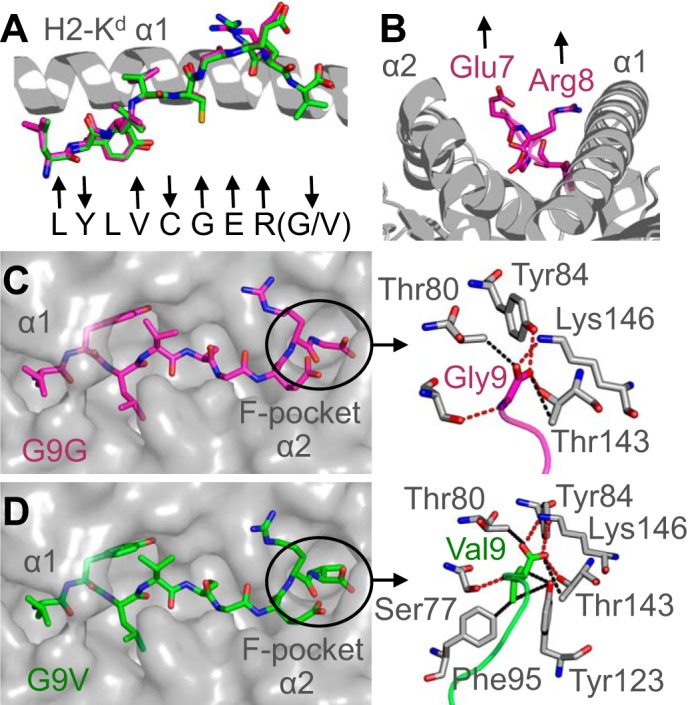
Interactions between the peptide C terminus and the MHC binding groove determine pMHC stability. A, superposition of the G9G peptide (magenta sticks) and G9V peptide (green sticks). The H-2Kd α1 domain is shown in a gray schematic. Arrows below the peptides indicate whether each residue is positioned away from the binding groove for potential TCR contact (up arrow), a primary or secondary anchor (down arrow), or in between (no arrow). B, peptide residues Glu-7 and Arg-8 (magenta sticks) bulge furthest away from the MHC groove (gray schematic). C, H-2Kd binding groove is shown in a gray surface representation demonstrating the extended conformation of the G9G peptide (magenta sticks). Right, the interactions between the C terminus of the G9G peptide (magenta sticks) and the MHC F-pocket (gray sticks). D, the H-2Kd binding groove is shown in a gray surface representation demonstrating the extended conformation of the G9V peptide (green sticks). Right, interactions between the C terminus of the G9V peptide (green sticks) and the MHC F-pocket (gray sticks).
