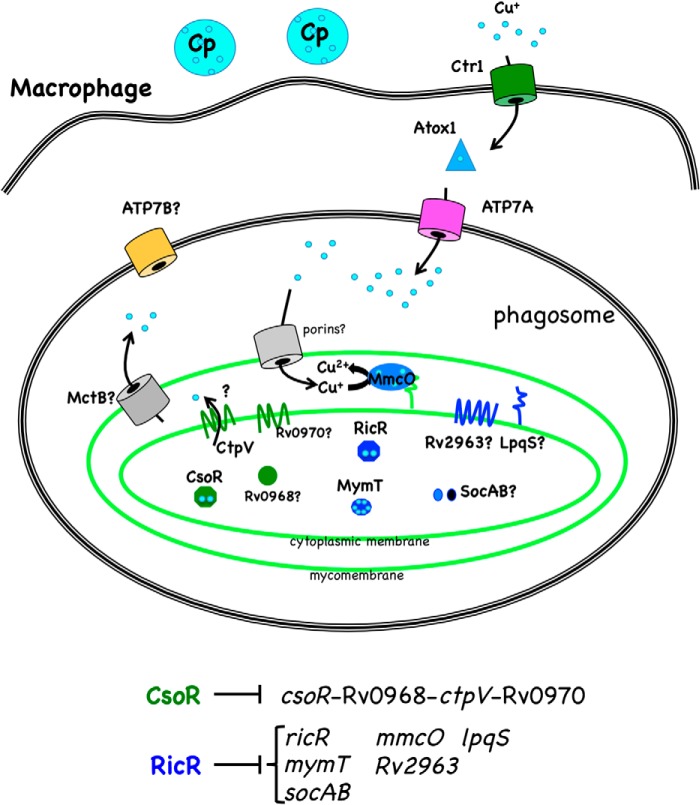
FIGURE 1.
M. tuberculosis and macrophage Cu homeostasis pathways. M. tuberculosis is found predominantly in phagocytic cells such as macrophages. Infection of macrophages by M. tuberculosis and other pathogens can lead to the production of IFNγ, which induces expression of Ctr1 and ATP7A on the host side of the interaction. Cu homeostasis on the host side proceeds as follows. Cp is a multicopper oxidase that converts ferrous iron (Fe2+) to ferric iron (Fe3+) throughout the body, Ctr1 is a high-affinity Cu+ transporter that transfers Cu to Atox1, a cytosolic Cu+ chaperone (reviewed in Ref. 30). Cu+ can then be transferred to the P-type ATPase ATP7A, which can pump Cu+ into the phagosome. ATP7B is another P-type ATPase that is essential for normal mammalian Cu homeostasis but has not yet been implicated in microbial pathogenesis (reviewed in Ref. 31). On the pathogen side, M. tuberculosis (light green) has at least three independent Cu-responsive pathways as described in the text. The CsoR regulon gene products are in dark green, RicR regulon gene products are in dark blue, and proteins of unknown regulation are in gray. Cu ions are depicted in light blue.