FIGURE 7.
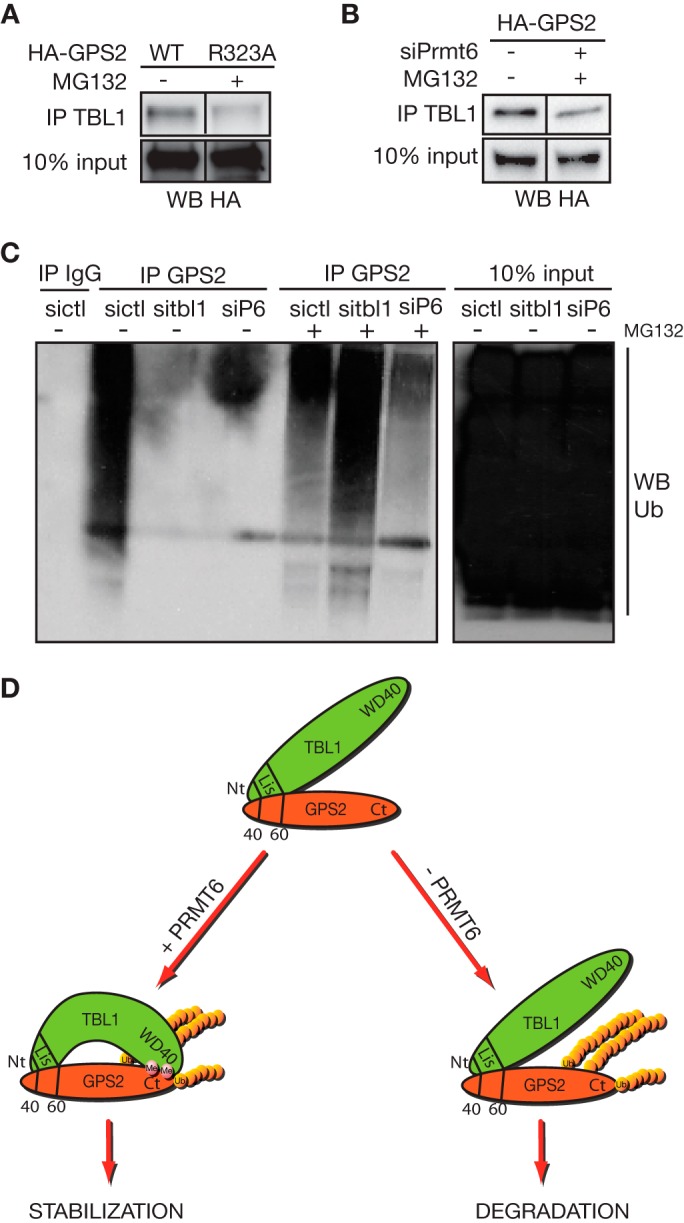
TBL1 protects polyubiquitinated GPS2 from proteasomal degradation. A, to compare equal amounts of GPS2 wild-type and R323A mutant in their ability to interact with TBL1, expression of the R323A mutant was rescued by MG132 (as shown in Fig. 5D), and the interaction was measured by coimmunoprecipitation (IP) in nuclear extracts from transiently transfected 293T cells. WB, Western blot. B, the same experimental approach is used to measure the effect of down-regulating PRMT6 via specific siRNA on the interaction between wild-type GPS2 and TBL1. In this case, expression of HA-GPS2 is rescued by MG132 when cells are depleted of PRMT6 (as shown in Fig. 5G). C, the polyubiquitination of GPS2 in absence of TBL1 or PRMT6 is assayed in 293T cells transfected with the specific siRNAs by GPS2 immunoprecipitation and Western blot against ubiquitin (Ub). D, proposed model of GPS2 alternative regulation by methylation and ubiquitination. GPS2 stability in the nucleus is strictly dependent on its interaction with the exchange cofactor TBL1. TBL1 direct interaction with GPS2 through the N-terminal domains of both proteins is further strengthened by regulated interaction between the respective C-terminal domains. This secondary interaction is regulated by PRMT6-mediated methylation of Arg-312/323 and is required for preventing GPS2 degradation. Ubiquitination of GPS2, as mediated by the E3 ligase Siah2, is not abrogated when the TBL1 protective role is removed, but degradation of polyubiquitinated GPS2 by the 26S proteasome is impaired under these conditions.
