FIGURE 4.
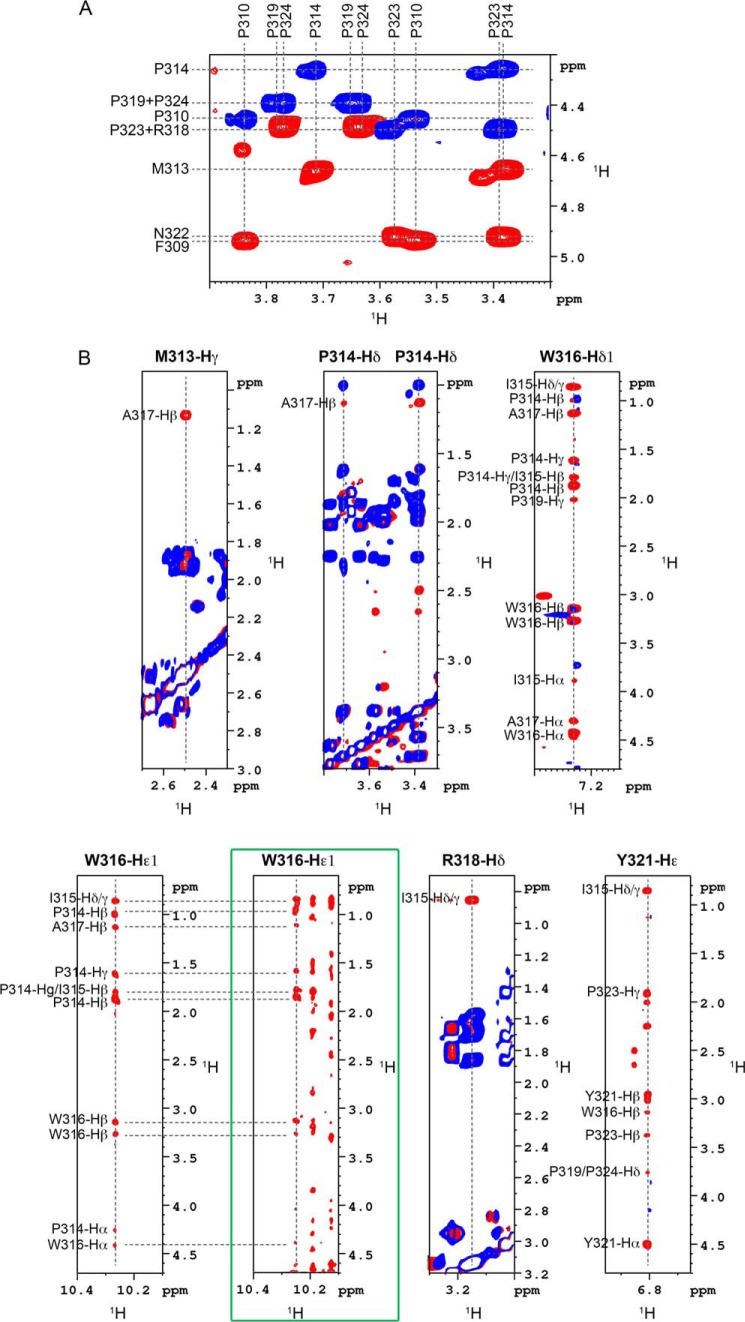
Definition of the structural element identified in NS5A-D2. A, isomeric state of proline residues in PepD2-WT. The figure corresponds to the superimposition of 1H,1H TOCSY (in blue) and 1H,1H NOESY (400-ms mixing time) (in red) spectra recorded on PepD2-WT (1 mm) and centered on the proline Hα/Hδ region. The NOE cross-peaks between the Hδ of all proline residues (i.e. Pro310, Pro314, Pro319, Pro323, and Pro324) and the Hα of their respective i − 1 residue indicate that all the proline residues are mainly in trans conformation. B, typical NOE contacts that define the structural element identified in NS5A-D2. Each panel corresponds to the superimposition of 1H,1H TOCSY (blue) and 1H,1H NOESY (400-ms mixing time) (red) spectra recorded on PepD2-WT at 600 MHz. These NOE contacts have been used as experimental distance constraints to calculate the structure of PepD2-WT. The green box highlights the NOE contacts recorded on the full-length 15N-labeled NS5A-D2 domain via a 15N NOESY HSQC experiment. The NOE pattern in the full-length domain is similar to that of the PepD2-WT peptide.