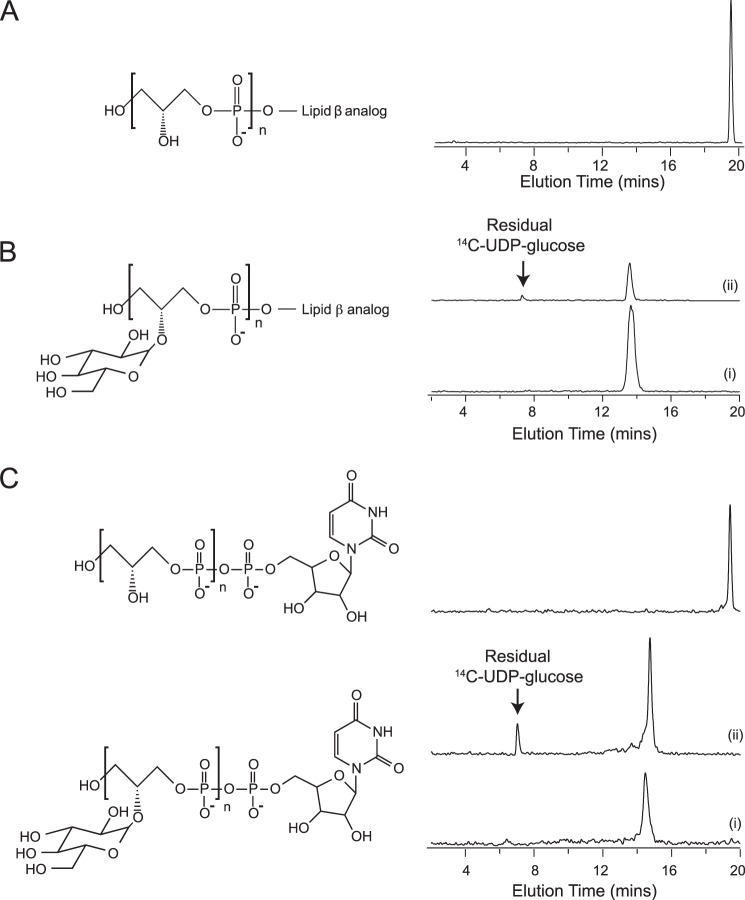
FIGURE 1.
Characterization of WTA analogs. Analog structures are shown next to radioactive chromatograms for A, [14C]glycerol lipid ϕ.40 analog; B, glycosylated lipid ϕ.40 analog with 14C incorporated into glycerol (i) and glucose (ii) (prepared by the inclusion of 0.42 mCi of UDP-d-[U-14C]glucose (250 mCi/mmol) to a TagE-catalyzed reaction containing non-radiolabeled lipid ϕ.40 analog); and C, CMP-poly(glycerol phosphate) WTA analogs. Polymerization of glycerol phosphate onto CMP-glycerol was accomplished by incubation of CDP-glycerol (3.29 mm) with TagF (34), and glycosylation was performed as with the lipid ϕ.40 analog. 14C was incorporated by the inclusion of either 1 μCi of CDP-[U-14C]glycerol (98.7 mCi/mmol) or 0.45 mCi of UDP-d-[U-14C]glucose (250 mCi/mmol). Chromatograms with glycosylated CMP-poly(glycerol phosphate) show polymer with 14C incorporated as [14C]glycerol (i) or [14C]glucose (ii). Under identical chromatographic conditions, retention times for CMP-poly(glycerol phosphate) analogs were slightly increased compared with tridecyl-linked analogs.