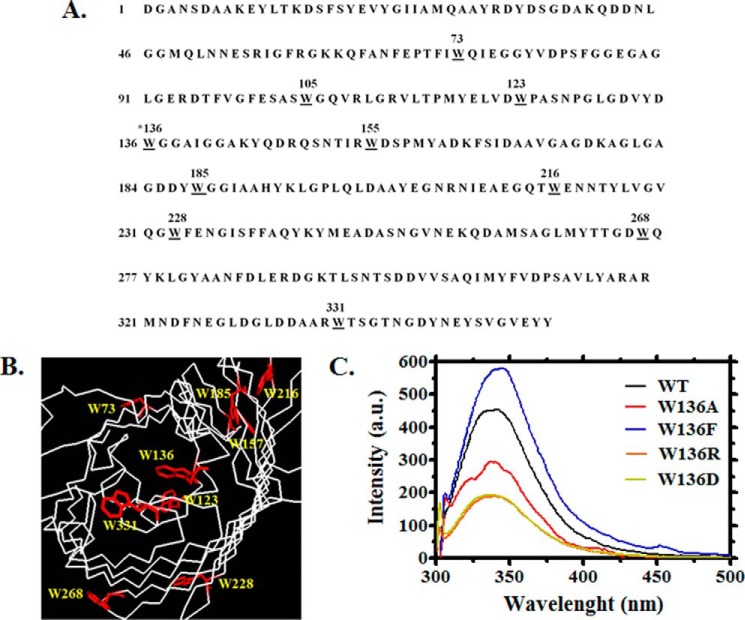
FIGURE 7.
Amino acid sequence and cross-section of the prospective three-dimensional structure of monomeric chitoporin from V. harveyi. A, amino acid sequence of VhChiP with all tryptophan residues underlined. B, amino acid residues that may be important for ion and sugar passages through the constriction zone of the VhChiP channel. C, example of the emission spectra from VhChiP WT and its Trp136 mutants, showing changes in fluorescence intensity caused by Trp136 mutations. The emission spectra were acquired at wavelengths between 300 and 550 nm using an excitation wavelength of 295 nm. The protein concentration used was 40 ng·μl−1 in 20 mm potassium phosphate buffer, pH 7.4, containing 0.2% (v/v) LDAO.