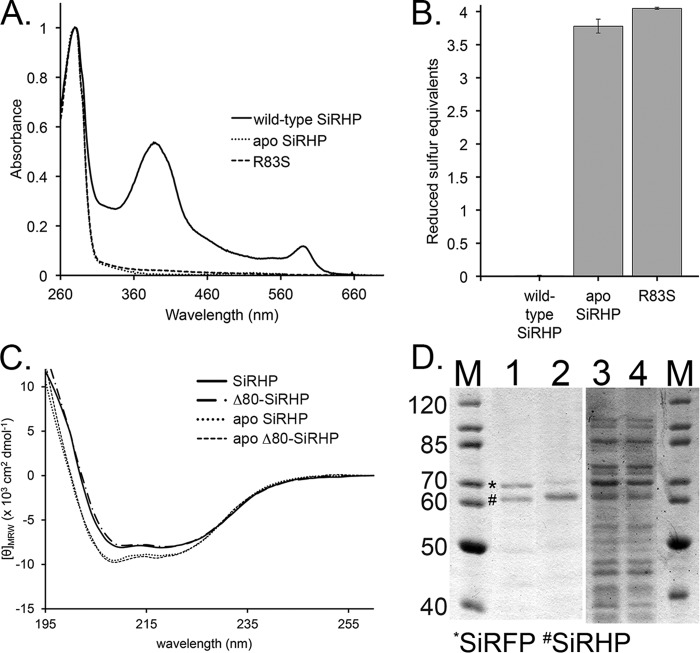
FIGURE 2.
Analysis of apo-SiRHP. A, UV-visible spectra of wild-type SiRHP (solid line), Δ60-SiRHP (short dashed/dotted line), and Δ80-SiRHP (long dashed/dotted line) showing siroheme's characteristic absorbances at 386 and 590 nm, absent in the apo-SiRHP (dotted line) and R83S (dashed line) spectra. B, Ellman's reagent test showing the absence of the Fe4S4 cluster in apo-SiRHP and R83S via detection of solvent-accessible thiols. The Fe4S4 cluster is coordinated by four cysteine residues, which, when absent, interact with Ellman's reagent to produce a signal of four reduced sulfur equivalents. C, CD spectra of wild-type SiRHP (solid line), Δ80-SiRHP (M-dash/dot), apo-SiRHP (dotted line) and apo-Δ80-SiRHP (dashed line). Apo-SiRHP and apo-Δ80-SiRHP have a similar change in secondary structure compared with SiRHP and Δ80-SiRHP upon the loss of siroheme cofactor. D, apo-SiRHP does not bind SiRFP in vivo. PageRuler protein markers (in kDa; Thermo Scientific) are shown in lanes 1 and 4. SiRFP (*) co-elutes with wild-type SiRHP (#) (lane 1) but is significantly reduced when co-precipitated with the R83S variant (lane 2). SiRFP is expressed strongly in both, shown by residual SiRFP in the unbound fractions (lanes 3 and 4). Residual SiRFP that co-elutes in the R83S variant probably corresponds to the small amount of R83S that is able to bind siroheme. Error bars, S.D.