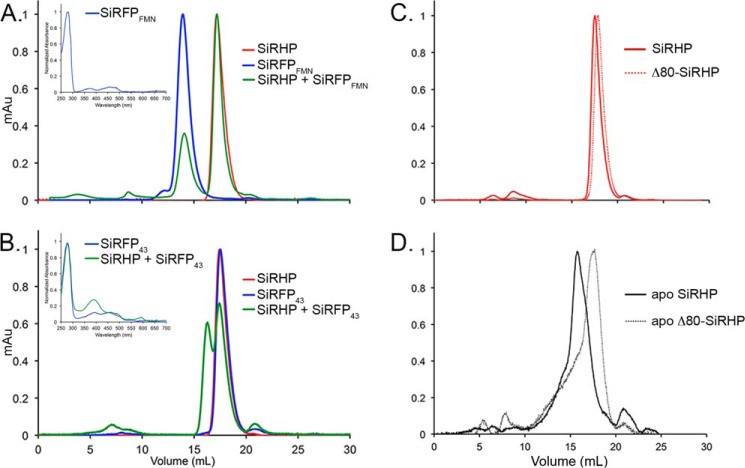
FIGURE 9.
SEC analysis of SiRFP truncations and Apo-SiRHP. A, SiRHP was mixed with C-terminally truncated SiRFP, an octomer of the FMN domains (SiRFPFMN). No higher-order structure was formed, indicating that the patch of amino acids 93–110 is not sufficient for forming a tight complex with SiRHP. Inset, UV-visible spectroscopy shows that SiRFPFMN binds flavin cofactor. B, SiRHP was mixed with N-terminally truncated SiRFP, a monomer of the FAD and NADPH domains (SiRFP43). A larger complex of higher molecular weight appears, indicating that the patch of amino acids 496–502 is sufficient for forming a tight complex. Inset, UV-visible spectroscopy shows that SiRFP43 binds flavin cofactor, and SiRHP/SiRFP43 shows a spectrum similar to that of SiR holoenzyme (Fig. 1A). C, wild-type SiRHP and Δ80-SiRHP run on SEC as tight peaks, corresponding to monomers of ∼64 kDa that differ slightly in size because of the N-terminal 80-amino acid truncation in Δ80-SiRHP. D, apo-SiRHP and apo-Δ80-SiRHP run at positions significantly different from those of their metallated counterparts because apo-SiRHP is a homotetramer, whereas apo-Δ80-SiRHP is a monomer. Both are broad, shouldered peaks indicative of loosely packed protein.