Figure 1. Interaction of malaria and Salmonella with macrophage iron.
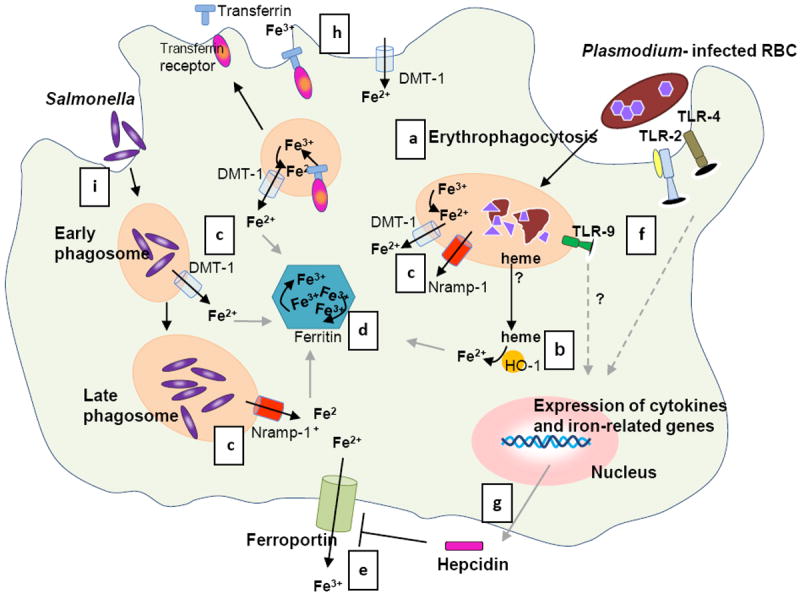
Phagocytosis of both uninfected and infected RBCs (a) is increased during the blood stage malaria infection [83], which results in degradation of the RBCs by proteolytic enzymes into heme. (b) HO-1 converts heme into iron (and carbon monoxide and biliverdin). (c) Excess iron is transported to the cytosol via phagosomal transporters DMT-1 and Nramp-1 [24] and further processed: (i) stored in ferritin (d), and (ii) used in metabolic processes or released from the cell via ferroportin (e) [26, 79]. (f) Meanwhile, parasite products activate the innate immune system via Toll Like receptors (TLR) 2, 4 and 9 [84]. This systemic response during malaria induces hepatic hepcidin production; (e) hepcidin functions by blocking ferroportin [29, 78]. In addition, monocytes and macrophages also express hepcidin upon stimulation with various pro-inflammatory cytokines and parasitized RBCs [36-38], (g) which may result in autocrine ferroportin blocking. (h) In addition, inflammatory stimuli inhibit ferroportin and modulate cellular iron uptake by DMT-1 and transferrin receptor [36, 39]. As a consequence of these processes iron is sequestered in macrophages. (i) Salmonella enters the cell via endocytosis and proliferates in phagosomes. Nramp-1 expression is required to control Salmonella growth by depleting the phagosome of iron (c) [46]. In a co-infection, Salmonellae spp. may benefit from the increased cellular iron induced by a malaria infection and establish an infection. Whether both pathogens reside in the same macrophage during invasive NTS infection and malaria as depicted in the figure is unknown. Illustration by A. Kartikasari.
