Abstract
Summary
We assessed the potential for countermeasures to lessen the loss of bone calcium during bed rest. Subjects ingested less calcium during bed rest, and with artificial gravity, they also absorbed less calcium. With exercise, they excreted less calcium. To retain bone during bed rest, calcium intake needs to be maintained.
Introduction
This study aims to assess the potential for artificial gravity (AG) and exercise (EX) to mitigate loss of bone calcium during space flight.
Methods
We performed two studies: (1) a 21-day bed rest (BR) study with subjects receiving 1 h/day AG (n=8) or no AG (n=7) and (2) a 28-day BR study with 1 h/day resistance EX (n=10) or no EX (n=3). In both studies, stable isotopes of Ca were administered orally and intravenously, at baseline and after 10 days of BR, and blood, urine, and feces were sampled for up to 14 days post dosing. Tracers were measured using thermal ionization mass spectrometry. Data were analyzed by compartmental modeling.
Results
Less Ca was absorbed during BR, resulting in lower Ca balance in BR+AG (−6.04±3.38 mmol/day, P=0.023). However, Ca balance did not change with BR+EX, even though absorbed Ca decreased and urinary Ca excretion increased, because endogenous excretion decreased, and there was a trend for increased bone deposition (P=0.06). Urinary N-telopeptide excretion increased in controls during BR, but not in the EX group. Markers of bone formation were not different between treatment groups for either study. Ca intake decreased during BR (by 5.4 mmol/day in the AG study and 2.8 mmol/day in the EX study), resulting in lower absorbed Ca.
Conclusions
During BR (or space flight), Ca intake needs to be maintained or even increased with countermeasures such as exercise, to enable maintenance of bone Ca.
Keywords: Biochemical markers of bone turnover, Exercise, Microgravity, Nutrition, Space flight
Introduction
Recent advances have documented the ability of resistance exercise and consuming enough calories to counteract bone mineral density loss during space flight [1, 2], but further questions remain. Resistance exercise protocols in space flight [1] or bed rest [3, 4] lead to increased bone remodeling, with increased bone formation along with the increase in bone resorption that is associated with weightlessness. Optimization of exercise and nutritional protocols may enhance these effects, and other potential countermeasures may be required for situations where these types of exercises are not available, due to space vehicle constraints, or simply in the event of equipment breakdown.
With respect to exercise, many types of regimens have been proposed and tested, including aerobic and resistance devices and combinations of these regimens [3–5]. From a nutritional perspective, dietary modifications have been proposed to mitigate bone loss [6], including lowering sodium intake [7], optimizing animal protein to potassium ratio [8], and increasing intake of omega-3 fatty acids [9]. Protein and amino acid supplementation has long been advocated as a countermeasure to muscle loss during space flight, but results have been equivocal at best [10] and are continually confounded by the interrelationship of these supplements with energy provision alone. Furthermore, amino acid supplementation has been shown to exacerbate bone resorption in bed rest [11], likely related to sulfur-containing amino acids and effects on acid-base balance [6, 8, 12].
Another countermeasure that has been described both in fictional accounts of space exploration and in discussion of potential future missions is the use of artificial gravity (AG) [13]. Implementation of AG would include either rotation of the entire space vehicle or episodic treatment of individual crewmembers in a smaller centrifuge. A vast literature exists on studies of bone health in unit gravity on Earth, and a growing field of literature describes the effects of extended periods of microgravity [14, 15], yet little is known about intermittent periods of gravity induced in subjects exposed to real or simulated weightlessness (such as bed rest).
In this paper, we report the results from two separate bed rest studies and evaluate the effects of exercise and artificial gravity countermeasures on calcium homeostasis and kinetics and on bone biochemical markers. The overall aim was to determine the ability of each countermeasure to mitigate the loss of calcium from bone in a ground-based analog of space flight.
Methods
The data reported here are from two larger studies of countermeasures for bone loss during bed rest: one study (AG) using artificial gravity and the other [exercise (EX)] using resistance exercise with timed amino acid supplementation. Other results from these studies have been published [16, 17]. Here, we report the tracer kinetic studies and bone marker data from the EX study.
Subjects
AG study
Fifteen healthy males participated in the study and were assigned to one of two groups: control (CON; n=7, mean age 27±2 years) and AG (n=8, 31±3 years). Subject characteristics and related data are shown in Table 1.
Table 1.
Subject and study characteristics
| Study |
||||
|---|---|---|---|---|
| AG |
EX |
|||
| Control | Treatment | Control | Treatment | |
| n | 7 | 8 | 3 | 10 |
| Age (years) | 27±2 | 31±3 | 40±7 | 42±8 |
| Weight (kg) | 82±8 | 81±9 | 92±2 | 87±6 |
| BMI (kg/m2) | 26±2 | 26±3 | 25±5 | 26±2 |
| Energy intake (kcal/kg BW) | ||||
| Pre-bed rest | 36±1 | 36±2 | 32±4 | 30±2 |
| Bed rest | 29±1 | 29±1 | 24±5 | 25±5 |
Data are mean ± SD
EX study
Thirteen healthy males participated in the tracer kinetic study and were assigned to one of two groups: CON (n=3, aged 40±7 years) and EX (n=10, 42±8 years). The original study design was more balanced, but the calcium kinetic protocol reported here was a late addition, and all available data are reported herein. Subject demographics are shown in Table 1.
Study design
AG study
Details of this study have been described elsewhere [16, 18, 19]. Briefly, the study consisted of a 12-day baseline period followed by a 21-day period of −6° head-down-tilt bed rest and a 7-day period of post-bed rest recovery (Fig. 1a). During the bed rest period, all subjects were transported daily on a −6° head-down-tilt gurney to the centrifuge facility, where they were transferred to the centrifuge arm (at 6° head-down tilt). Bed rest (BR)+AG subjects received 1 h of centrifugation, whereas control subjects did not. Artificial gravity was produced by rotating the subjects on a centrifuge arm with a radius of 3.0 m. Energy intake was restricted (Table 1) during bed rest, to account for reduced energy expenditure, but was adjusted as needed to maintain body weight (to within±3 % of bed rest day 3 weight).
Fig. 1.
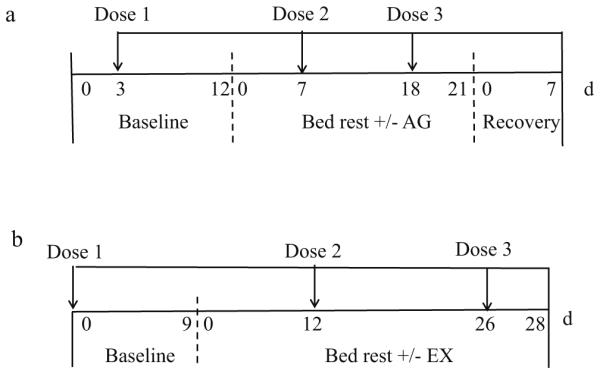
Study design showing the length of baseline, bed rest, and recovery periods for the AG study (a) and EX study (b). The times of the three doses of tracer administration are indicated by the arrows above the timeline for each study
EX study
Details of this study have been published [17, 20]. The study consisted of a 9-day baseline period followed by a 28-day period of horizontal bed rest. This was followed by 14 days of post-bed rest recovery, but no kinetic data were collected during the recovery period (Fig. 1b). Energy intake was restricted during bed rest (by an average of 8 % below estimated requirements, which are reduced during bed rest due to inactivity) to induce weight loss (Table 1) and to mimic some of the earlier observations from space flight [17]. All subjects received a supplement consisting of 15 g of essential amino acids plus 35 g sucrose, dissolved in 500 mL water daily during bed rest. This supplement added an additional 200 kcal/day to their energy intake. Three control subjects participated in the kinetic studies; they received the amino acid/sucrose supplement but did not exercise. Among the exercisers, some received the supplement 5 min before exercise, and some received the supplement 3 h after exercise. Given the small sample size and lack of differences between these treatments, for this study, all exercisers were combined into one group (n=10 total). Subjects undertook supervised exercise sessions for 1 h/day, 6 days/week. Details of the exercise sessions have been described elsewhere [17]. Briefly, upper- and lower-body exercises (alternate days) were performed in the horizontal position using a modified Shuttle Accel machine (Contemporary Design, Glacier, WA), with resistance provided by elastic cords attached from the stationary frame to the sliding carriage.
Tracers and administration
Stable isotopes of Ca, as CaCO3 (Trace Sciences International, Inc., Pilot Pont, TX), were prepared and administered as described previously [21, 22].
AG study
Stable isotopes of Ca were administered to each subject three times during the study (Fig. 1a). For the baseline session (dose 1, 9 days before bed rest), 46Ca (25 μg) was given orally, and 48Ca (1 mg, as CaCl2, in a sterile isotonic solution) was injected intravenously. For the second tracer session (dose 2 on day 7 of bed rest), 46Ca (75 μg) was given orally, and 44Ca (6 mg, as CaCl2, in a sterile isotonic solution) was administered intravenously. For the third session (dose 3 on day 18 of bed rest), an 8-mg dose of 42Ca was given orally. The oral doses were given with a 250-mg Ca carrier to approximate the load of Ca typically ingested in a meal.
EX study
The tracers administered were the same as in the AG study, but in the EX study, the times of administration were 9 days before bed rest and on days 12 and 26 of bed rest (Fig. 1b).
Sampling
AG study
After tracer administration of dose 1, blood was sampled at 1, 5, and 12 h and at 1, 3, 5, and 9 days. After administration of dose 2, blood was sampled at 1, 5, and 12 h and at 1, 3, 5, and 7 days. After dose 3, blood was sampled at 1, 5, and 12 h and at 1, 3, 5, 7, and 11 days. Complete urine collections were made for 16 days after dose 1, for 11 days after dose 2, and for 11 days after dose 3. Complete fecal collections were made for 14, 9, and 10 days after each dose, respectively.
EX study
After tracer administration of dose 1, blood was sampled at 1, 3, and 9 h and at 1, 3, 5, 7, 9, and 13 days; after dose 2, it was sampled at 1, 3, and 9 h and at 1, 3, 5, 7, 9, and 11 days; and after dose 3, it was sampled at 1, 3, and 9 h and at 1 day. Complete urine collections were made after doses 1 and 2 for 14 days and after dose 3 for 2 days. Complete fecal collections were made for 5 days after doses 1 and 2, but no feces were collected after dose 3.
Biosample Analyses
Urine, serum, and fecal samples were analyzed for stable isotopes using thermal ionization mass spectrometry as described previously [23, 24]. Blood and urine biochemical markers of bone resorption, bone formation, and minerals were measured as previously described [16].
Calcium kinetic analysis
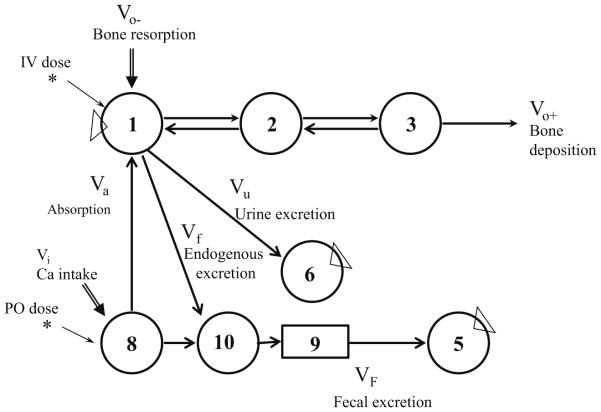
Data were analyzed by compartmental modeling using the WinSAAM software [25] with a previously reported model (Fig. 2) consisting of three compartments and pathways representing Ca in the vascular space (compartment 1), extravascular space (compartment 2), and exchangeable bone Ca (compartment 3). Ca is lost from the central compartment when it is deposited into bone or excreted into urine or feces.
Fig. 2.
A model of calcium metabolism in humans. Circles represent compartments, numbers in circles represent compartment numbers, arrows represent movement of calcium between compartments, and double-shafted arrows represent entry of calcium by way of the diet (Vi) or bone resorption (Vo−). The asterisks indicate entry of tracer, and triangles indicate sampled compartments. Compartment 1 contains blood, 2 extravascular space, and 3 exchangeable calcium on bone. Compartment 5 represents feces, and 6 urine, and 8 and 10 sites in the intestine. The box labeled 9 represents a delay in movement of calcium in the lower intestine. Fractional absorption was calculated as L(1,8)/(L(1,8)+L(10,8))
The model was fitted to the data by minimizing the deviations using the least squares regression procedure in WinSAAM. Fractional transfer, L(i,j) (which refers to transfer into compartment i from compartment j per unit time); compartment mass, M(i) (in mg); and rates of calcium transport, R(i,j) (mg per unit time), as the product of mass and the fractional transfer coefficient, were calculated from the model as described [26]. Fractional absorption was calculated as
Fractional absorption = L(1, 8)/(L(1, 8) + L(10, 8))
Calculation of absorption was based on an oral tracer (administered with breakfast, which contained 6.25 mmol Ca) and on the total daily dietary intake (which contained 23–38 mmol Ca/day). The time change feature in WinSAAM was used to allow parameter values to change at the start and end of bed rest and to add tracer at the time of doses 2 and 3. For the model to fit the data during bed rest, it was necessary to allow some parameters of the model to differ from the baseline period. These parameters were absorption, L(1,8); urine excretion, L(6,1); endogenous excretion, L(10,1); and in some subjects, L(2,3). Rates of calcium absorption (Va), fecal excretion (VF), urinary excretion (Vu), endogenous excretion (Vf), bone deposition (Vo+), bone resorption (Vo−), and bone balance (Vo+−Vo−) were calculated using the model (Fig. 2).
Statistical analysis
Values are reported as mean ± SD. Differences between groups for the calcium kinetic variables were compared by Student’s t test, and differences between treatments were compared with Student’s paired t test. Differences were considered significant if P<0.05.
Data for urine and blood biochemical markers from the EX study, in their original form, were analyzed by repeated measures ANOVA, with time as the repeated factor and treatment as the grouping factor. When the main effect was found to be significant, a post hoc Bonferroni t test was performed to determine the difference from BR-2 (urine) or BR-9 (blood). Differences were considered significant if P<0.05. Statistical analyses were performed using SigmaPlot software version 12.0 (Systat Software, Inc., San Jose, CA).
Results
Calcium kinetics
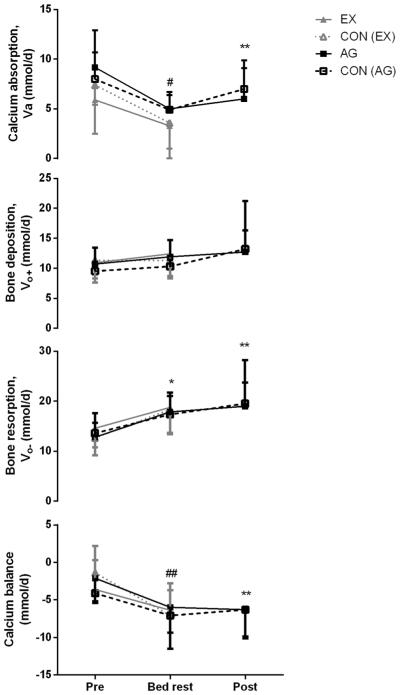
In the AG study, there were no differences in kinetic parameters between control and AG groups during baseline, BR, or post-BR (data not shown), and effects of bed rest in each group were similar, although not always identical with respect to statistical significance. In the control group, during bed rest, a significant decrease in Ca intake and also in the rate of calcium absorption (Va) and an increase in bone resorption (Vo−) occurred, but no significant change occurred in Ca balance (Fig. 3, Online Resource 1). The same changes (lower Ca intake, lower Ca absorption, and higher bone resorption) were observed in the AG subjects during bed rest relative to pre-bed rest, but fractional absorption from the diet was also lower, and in these individuals, bone calcium balance decreased. The control subjects showed a similar trend for these two changes (in fractional absorption and bone calcium balance), but this did not reach statistical significance (Online Resource 1).
Fig. 3.
Calcium kinetics (Ca absorption, bone deposition, bone resorption, and calcium balance) during and after bed rest with (EX) and without exercise [CON(EX)] and with (AG) and without artificial gravity [CON(AG)]. Asterisk indicates significant difference from pre-bed rest in all four groups. **P<0.05, significantly different from pre-bed rest in the AG group; #P<0.05, significantly different from pre-bed rest in the CON(AG), AG, and EX groups; ##P<0.05, significantly different from pre-bed rest in the CON(EX) and AG groups
During recovery, the control subjects’ calcium kinetic data did not differ from baseline except that both Ca intake and urinary excretion (Vu) were higher during recovery (Online Resource 1). In the AG group, in addition to the changes seen in controls, fractional absorption and total calcium absorbed from diet were lower, and fecal excretion and bone resorption were significantly higher than baseline (but were not different from the bed rest period). As a result, Ca balance during recovery was significantly lower than baseline in the AG group, although the control and AG groups had similar balance, about −7.5 mmol/day (Online Resource 1).
In the exercise study, there were no differences between control and EX in the pre-BR or BR periods (data not shown), but relative to baseline, during BR control, subjects had lower fractional absorption, increased bone resorption, and lower Ca balance (Fig. 3, Online Resource 2). The EX subjects had lower Ca intake during bed rest relative to baseline, decreased Ca absorption, increased urinary calcium excretion, decreased endogenous excretion, and increased bone resorption. No change occurred in calcium balance during bed rest, although bone deposition tended to increase (P<0.058) (Fig. 3, Online Resource 2). Fractional absorption estimated during late bed rest (day 26) for controls (0.220±0.016) and EX subjects (0.274±0.090) did not differ between groups or from baseline or day 12 of BR within each group (data not shown). Similar to the AG study, bone calcium balance during bed rest was similar in these groups as well, about −7 mmol/day (−300 mg/day) (Fig. 3, Online Resource 2).
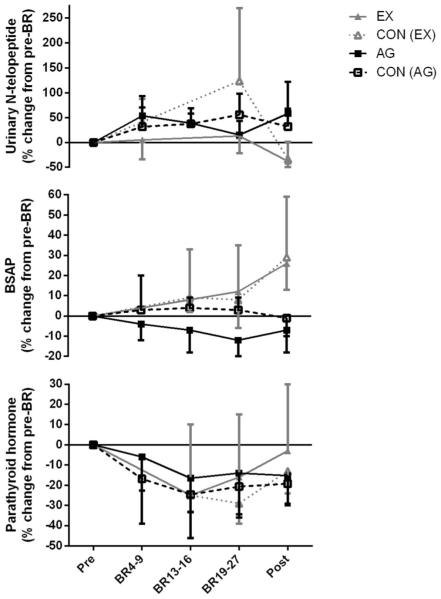
Biochemical analyses
Biochemical data from the AG study have been previously reported [16] but are shown in Fig. 4 for comparison. In general, markers of bone resorption increased during BR, but there was no difference between controls and AG subjects (Fig. 4) [16]. In the EX study, markers of bone resorption, deoxypyridinoline (DPD) and helical peptide, increased in both controls and EX subjects during bed rest (Online Resource 3). N-telopeptide (NTX) increased in the controls, but not in the EX group (P<0.001). Urinary calcium increased in both groups by bed rest day 5 and remained elevated for the duration of bed rest but returned to baseline 10 days after reambulation (Online Resource 3).
Fig. 4.
Urinary N-telopeptide, serum bone-specific alkaline phosphatase (BSAP), and serum parathyroid hormone, before, during, and after bed rest. To simplify the figure, the x-axis time points were averaged into the groupings presented, but all statistical analyses were performed on the raw data that are presented in the online resources
Although 25-hydroxy vitamin D was not different between groups, 1,25-dihydroxy vitamin D decreased during bed rest in both groups (Online Resource 4, P<0.01). Bone-specific alkaline phosphatase was unchanged during bed rest but increased in both groups after bed rest (P<0.001) (Fig. 4, Online Resource 4). Insulin-like growth factor 1 (IGF-1) was elevated in both groups during bed rest but returned to baseline during recovery (P<0.001) (Online Resource 4).
Discussion
The calcium tracer kinetic technique provides a valuable insight into bone and calcium metabolism in these types of studies. It is intriguing that bone calcium balance, perhaps the most critical aspect of these data, proves to be remarkably consistent across studies. In the two bed rest studies here, we report group mean calcium balances of −7.1, −7.1, −6.4, and −6.0 mmol/day during bed rest. Our earlier calcium kinetic studies from six astronauts during space flight documented an average calcium balance of −5.9 mmol/day (−236 mg/day) [21].
During bed rest, we observed that the control and AG subjects had similar changes in calcium kinetics. Furthermore, in the AG group, during recovery, calcium absorption remained lower and urinary Ca excretion higher, bone resorption was elevated, and Ca balance was significantly lower relative to paired baseline data, whereas in controls, these values had returned to pre-bed rest levels. This suggests a delay in return to pre-bed rest calcium metabolism, and AG in effect imposed a higher calcium requirement on the BR subjects that was not met by dietary Ca intakes of 1,200–1,500 mg/day, thus resulting in accelerated bone loss relative to the controls. These kinetic differences evident between the AG and control groups were not detected using urinary markers of bone resorption, indicating that these biomarkers are likely not as sensitive as the kinetic/modeling approach, despite their relative ease of data collection and analysis. The nature and cause of this effect are unknown, but the effect highlights the need to monitor physiological changes not only during bed rest (or space flight) but also after.
In the exercise study, calcium balance decreased in the controls during bed rest, and similar trends occurred in the EX group. Calcium balance in the EX group seemed to be slightly better than that in controls, in part because of decreased endogenous fecal Ca excretion. These results suggest that exercise triggered a homeostatic response to maintain calcium balance. As has been seen in other bed rest and space flight studies, the EX group tended to increase their rate of bone Ca deposition [1, 3, 4]. Supportive of the kinetic data, which revealed increased bone resorption in both groups, two of the three measured urinary collagen cross-link markers were significantly increased in both groups during bed rest. The third, N-telopeptide, was elevated in controls by bed rest day 19 and remained elevated throughout bed rest but did not change in the EX group. Bone formation markers were equivocal, with the bone-specific alkaline phosphatase (BSAP) response not being significantly different between groups, and so were osteocalcin and IGF-1. These data, as in the AG study, suggest that the systemic markers are likely not as sensitive as the kinetic measurements.
Bed rest studies are a key step in the evaluation of countermeasures to the negative effects of space flight. Although such studies are difficult to design, implement, and fund, they provide critical information from a space flight analog about the potential effectiveness of countermeasures before they are tested during actual space flight, where resources and logistics are even more restricting. We report here the results of two such studies, to evaluate physical countermeasures designed to stimulate bone formation and to counteract disuse-induced bone loss. On Earth, exposed to unit gravity, muscle force and bone strain help to maintain bone mass, and in weightlessness, exposed to skeletal unloading, bone is lost with muscle. However, we have little knowledge of the extent to which gravity must be diminished to produce bone loss.
Limitations of the study
A key limitation of the exercise study is the small sample size for the controls (n=3), and this small number likely prevented the detection of significant differences between groups. Although the main study was more balanced in design and size [17, 20], the calcium kinetic protocol was a late addition, and all available data are reported herein. Additionally, although it would have been informative to evaluate the effects of the amino acid/carbohydrate supplement on calcium metabolism in detail, cost limitations would not allow the implementation of a fully balanced design. For similar reasons, the studies were conducted on male subjects only. Looking at sex differences under these specific conditions would be beneficial, although we have data documenting that there are no sex differences in bone loss or renal stone risk during flight [2] or bed rest (Smith et al., unpublished observations).
Both studies were designed to restrict energy intake during bed rest (to maintain body weight in the AG study and to mimic decreased intake in some astronauts in the exercise study). An unintended consequence was that calcium intake was also decreased during bed rest (by 14 % in the AG study and 11 % in the exercise study), possibly contributing to lower absorbed calcium. We conclude that during bed rest (or space flight), calcium intake needs to be maintained or even increased, when countermeasures such as exercise are implemented, to ensure the ability to maintain bone calcium. Insufficient calcium intake, regardless of gravitational field, is detrimental to bone health.
Summary and conclusions
Many factors affect bone metabolism, including in a broad sense gravity, nutrition, and exercise. From the data reported here and in earlier reports, it is clear that a loss of 0.25 g calcium per day while ingesting Ca intakes of 1,000–1,200 mg/day cannot be sustained over multi-year exploration missions and that effective countermeasures are required.
Recent studies have documented that adequate nutritional intake, including energy and vitamin D status, and a combination of aerobic and heavy resistance exercise can protect bone mineral density during 6-month space missions [1, 2]. Although the exercise countermeasure reported here did not maintain bone density similar to what has been seen in space flight, we know of a few factors that may have influenced this. First, the bed rest subjects were energy-restricted and lost body mass as a result [17]. Energy restriction is known to have negative effects on bone metabolism [27], and we maintain that this is a key element of the success of nutritional intake and exercise in protecting bone density on the ISS. Furthermore, the space flight studies compared two exercise devices: an interim resistance exercise device (iRED) and an advanced resistance exercise device (ARED). The device reported in this paper is based on elastic bands providing resistance, much like the iRED. The exercise in the present study failed to have an impact on bone formation, as evaluated with calcium kinetic or blood biochemical markers. Increased bone formation is a hallmark of successful exercise and bed rest/space flight studies, as we have observed on several occasions [1–4]. Thus, the data presented here fit with and complement earlier research.
The studies reported here provide novel information about bone and calcium metabolism during bed rest, with two types of physical countermeasures. The kinetic study reported here provides key details of bone and calcium metabolism during bed rest while still highlighting the need for more research to optimize nutrition and exercise regimens to protect and preserve bone in situations of disuse, such as bed rest and space flight. Further research is required to address these questions.
Supplementary Material
Acknowledgments
We thank the subjects for their time and willingness to participate in these studies. We thank the staff of the UTMB Institute for Translational Sciences Clinical Research Center for their assistance with the AG study and the Tufts University GCRC for their support in conducting the exercise study. We thank the staff of the U.S. National Aeronautics and Space Administration (NASA) Johnson Space Center Nutritional Biochemistry Laboratory for their assistance in processing and analyzing the samples and in all aspects of carrying out this project. We thank Jane Krauhs for editorial assistance. The AG study was funded in part by the NASA Flight Analogs Project of NASA’s Human Research Program and in part by grant 1UL1RR029876-01 from the National Center for Advancing Translational Sciences, National Institutes of Health. The exercise study was supported by the National Space Biomedical Research Institute (NSBRI) through NCC 9-58, by agreement 58-1950-9-001 from the U.S. Department of Agriculture’s Agricultural Research Service, and by a grant M01-RR-000054 from the National Institutes of Health GCRC.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00198-014-2754-x) contains supplementary material, which is available to authorized users.
Conflicts of interest None.
Ethical standards All bed rest protocols complied with the World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects—and were reviewed and approved by the National Aeronautics and Space Administration (NASA) Johnson Space Center Institutional Review Board (IRB) or its predecessor, the Committee for the Protection of Human Subjects, and either the University of Texas Medical Branch (UTMB) IRB (AG study) or the IRB at the Tufts University Medical Center, Boston, MA (EX study). All subjects received verbal and written explanation of the protocol and provided written informed consent.
Contributor Information
S. M. Smith, NASA Lyndon B. Johnson Space Center, Attn: Mail Code SK3, 2101 NASA Parkway, Houston, TX 77058, USA
C. Castaneda-Sceppa, Northeastern University, Boston, MA, USA
K. O. O’Brien, Cornell University, Ithaca, NY, USA
S. A. Abrams, Baylor College of Medicine, USDA/ARS Children’s Nutrition Research Center, Houston, TX, USA
P. Gillman, EASI, Houston, TX, USA
N. E. Brooks, University of Stirling, Scotland, UK
G. J. Cloutier, Northeastern University, Boston, MA, USA
M. Heer, University of Bonn, Bonn, Germany
S. R. Zwart, Universities Space Research Association, Houston, TX, USA
M. E. Wastney, Metabolic Modeling, West Lafayette, IN, USA
References
- 1.Smith SM, Heer MA, Shackelford L, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–1906. doi: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Zwart SR, Heer M, Hudson EK, Shackelford L, Morgan JL. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2185. doi:10.1002/jbmr.2185. [DOI] [PubMed] [Google Scholar]
- 3.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–129. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Zwart SR, Heer M, Lee SMC, Baecker N, Meuche S, Macias BR, Shackelford LC, Schneider S, Hargens AR. WISE-2005: supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone. 2008;42:572–581. doi: 10.1016/j.bone.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Armbrecht G, Belavy DL, Gast U, Bongrazio M, Touby F, Beller G, Roth HJ, Perschel FH, Rittweger J, Felsenberg D. Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: biochemical markers of bone metabolism. Osteoporos Int. 2010;21:597–607. doi: 10.1007/s00198-009-0985-z. [DOI] [PubMed] [Google Scholar]
- 6.Zwart SR, Smith SM. The impact of space flight on the human skeletal system and potential nutritional countermeasures. Intl Sport Med J. 2005;6:199–214. [Google Scholar]
- 7.Frings-Meuthen P, Buehlmeier J, Baecker N, Stehle P, Fimmers R, May F, Kluge G, Heer M. High sodium chloride intake exacerbates immobilization-induced bone resorption and protein losses. J Appl Physiol. 2011;111:537–542. doi: 10.1152/japplphysiol.00454.2011. [DOI] [PubMed] [Google Scholar]
- 8.Zwart SR, Hargens AR, Smith SM. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr. 2004;80:1058–1065. doi: 10.1093/ajcn/80.4.1058. [DOI] [PubMed] [Google Scholar]
- 9.Zwart SR, Pierson D, Mehta S, Gonda S, Smith SM. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-kappaB activation: from cells to bed rest to astronauts. J Bone Miner Res. 2010;25:1049–1057. doi: 10.1359/jbmr.091041. [DOI] [PubMed] [Google Scholar]
- 10.Stein TP, Blanc S. Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit Rev Food Sci Nutr. 2011;51:828–834. doi: 10.1080/10408398.2010.482679. [DOI] [PubMed] [Google Scholar]
- 11.Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM. Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol. 2005;99:134–140. doi: 10.1152/japplphysiol.01406.2004. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, Zwart SR, Kloeris V, Heer M. Nutritional biochemistry of space flight. Nova Science Publishers; New York: 2009. [Google Scholar]
- 13.Clément G, Bukley AP. Artificial gravity. Springer; New York: 2007. [Google Scholar]
- 14.Sibonga JD, Cavanagh PR, Lang TF, LeBlanc AD, Schneider VS, Shackelford LC, Smith SM, Vico L. Adaptation of the skeletal system during long-duration spaceflight. Clin Rev Bone Miner Metabol. 2008;5:249–261. [Google Scholar]
- 15.Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, Bakulin AV, Shackelford LC, LeBlanc AD. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41:973–978. doi: 10.1016/j.bone.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Zwart SR, Heer MA, Baecker N, Evans HJ, Feiveson AH, Shackelford LC, Leblanc AD. Effects of artificial gravity during bed rest on bone metabolism in humans. J Appl Physiol. 2009;107:47–53. doi: 10.1152/japplphysiol.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks N, Cloutier GJ, Cadena SM, Layne JE, Nelsen CA, Freed AM, Roubenoff R, Castaneda-Sceppa C. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol. 2008;105:241–248. doi: 10.1152/japplphysiol.01346.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren LE, Reinertson R, Camacho ME, Paloski WH. Implementation of the NASA Artificial Gravity Bed Rest Pilot Study. J Gravit Physiol. 2007;14:P1–P4. [PubMed] [Google Scholar]
- 19.Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, Inniss AM, Rice BL, Ericson K, Coburn S, Bourbeau Y, Hudson E, Mathew G, Dekerlegand DE, Sams CF, Heer MA, Paloski WH, Smith SM. Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol. 2009;107:54–62. doi: 10.1152/japplphysiol.91136.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks NE, Cadena SM, Vannier E, Cloutier G, Carambula S, Myburgh KH, Roubenoff R, Castaneda-Sceppa C. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve. 2010;42:927–935. doi: 10.1002/mus.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM, Wastney ME, O’Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J Bone Miner Res. 2005;20:208–218. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol. 1999;277:R1–R10. doi: 10.1152/ajpregu.1999.277.1.r1. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Wastney ME, Nyquist LE, Shih CY, Wiesmann H, Nillen JL, Lane HW. Calcium kinetics with microgram stable isotope doses and saliva sampling. J Mass Spectrom. 1996;31:1265–1270. doi: 10.1002/(SICI)1096-9888(199611)31:11<1265::AID-JMS419>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Abrams SA, Esteban NV, Vieira NE, Yergey AL. Dual tracer stable isotopic assessment of calcium absorption and endogenous fecal excretion in low birth weight infants. Pediatr Res. 1991;29:615–618. doi: 10.1203/00006450-199106010-00018. [DOI] [PubMed] [Google Scholar]
- 25.Stefanovski D, Moate PJ, Boston RC. WinSAAM: a windows-based compartmental modeling system. Metabolism. 2003;52:1153–1166. doi: 10.1016/s0026-0495(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 26.Wastney ME, Patterson BH, Linares OA, Greif PC, Boston RC. Investigating biological systems using modeling: strategies and software. Academic Press; New York: 1998. [Google Scholar]
- 27.Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19:1231–1240. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.