Abstract
Nocardia otitidis-cavarium is rarely isolated as an infectious pathogen in the western world. We report on a 71-year-old Caucasian man with membranous glomerulonephritis who presented with several seemingly unrelated clinical symptoms that, after laborious diagnostics, turned out to be caused by disseminated infection with N. otitidis-cavarium. This case highlights the variable clinical presentations that can occur in nocardial infections and underscores the need to search for rare pathogens in patients taking immunosuppressive medication.
Background
Nocardia of the order Actinomycetales, are weakly Gram-positive staining bacteria that, in their bacillary or coccoid forms, can form partially acid-fast branching filaments. Nocardial infections are rare, with an estimated incidence of approximately 2–3 per million a year in the USA and around 3–5 per million in France.1 Within the genus Nocardia, approximately 80–85% of infections are caused by Nocardia asteroides sensu stricto, while other species such as N. nova, N. farcinica, N. transvalensis and N. brasiliensis account for most other cases, with regional variation in their respective percentage.2 At least 50% of nocardial infections occur in patients with some form of immunosuppression, most notably after organ transplantation, and in patients with AIDS, intravenous drug abuse or an underlying malignancy.1 3 4 N. otitidis-cavarium is very rarely isolated as the infectious pathogen.5 It is associated with infections of traumatic wounds in normal hosts and is thought to be less pathogenic than other Nocardia spp.6 Searching the medical literature from 1966 to 2014 yielded only approximately 50 infections with N. otitidis-cavarium. None of these were described in the context of membranous glomerulonephritis (MGN) or presented with a disseminated intramuscular involvement.
Case presentation
A 71-year-old man was referred from his primary care physician with a preliminary diagnosis of peripheral arterial vascular disease. He had a history of smoking (70 pack-years), repair of an abdominal aortic aneurysm 9 years earlier and had developed diabetes after receiving steroids to treat idiopathic MGN 6 months before this admission. In the past, he had been treated for atrial fibrillation, hypertension, heart insufficiency due to aortic valve stenosis and mitral valve insufficiency, acute exacerbations of chronic obstructive lung disease, liver cirrhosis and upper gastrointestinal bleeding due to Mallory-Weiss lesions. He reported a maximum walking distance of 10–20 m limited by pain in his left upper leg and dyspnoea that had worsened over the last few weeks. When he initially presented with his MGN, our patient had progressive renal function decline and nephrotic range proteinuria. After a renal biopsy and after ruling out secondary causes for MGN, he was treated with steroids and three intravenous doses of cyclophosphamide. Subsequently, his renal disease was stable with a creatinine clearance of 40 mL/min and minimal proteinuria (100 mg/day) under low-dose prednisone (10 mg/day) combined with mycophenolate mofetil (MMF, 2×500 mg/day).
On examination, the patient had reduced breath sounds in the right upper lung field, a 3/6 systolic heart murmur over the aortic valve and a 5/6 systolic murmur over the mitral valve extending into the left axilla. He had a temperature of 38.5°C and reported recurrent fever during the last weeks as well as 6 kg weight loss over the last 3 months. Chest X-ray showed an opacified right upper lung lobe. The musculature of both his thighs appeared markedly atrophied and his left leg was painful to external rotation. On the lower part of the left sartorius muscle and in the middle of the vastus lateralis muscle, two tender, non-erythematous, non-overwarmed nodules could be palpated deeply subcutaneously.
Investigations
The patient's laboratory values on admission and during the subsequent hospital stays are given in table 1. A CT of the chest showed a homogenous opacification of the right dorsoapical pulmonary segment, multiple round nodules in the right upper and lower lobe with a maximum diameter of 15×15 mm, plus enlarged mediastinal lymph nodes (figure 1A, B). An angio-MRI of the pelvic and leg arteries showed several high-grade stenoses as well as two hypervascularised tumours in the left sartorius muscle and vastus lateralis muscle, and another one in the right vastus medialis muscle. A bronchoscopy was performed showing purulent secretions from the right upper lobe and severe chronic bronchitis. Cytology confirmed an acute inflammation with masses of neutrophils and yeast-like structures. Cultures were negative for bacteria including Mycobacterium tuberculosis, as was PCR for M. tuberculosis. Histology showed a fibrinous, purulent, partly necrotising pneumonia with abscesses and yeast-like structures.
Table 1.
Laboratory findings at decisive time points during our patient’s nocardial infection
| Laboratory test | 1. Admission | Diagnosis | 1. Discharge | Outpatient visit | 2. Admission | 2. Discharge | 3. Admission | 3. Discharge |
|---|---|---|---|---|---|---|---|---|
| RBC (106/µL) | 3.62 | 3.48 | 3.35 | 3.78 | 2.54 | 3.46 | 3.42 | 2.88 |
| Haemoglobin (g/dL) | 10.0 | 9.6 | 10.2 | 11.6 | 7.5 | 10.1 | 9.0 | 8.7 |
| Haematocrit (%) | 32.2 | 31.6 | 30.9 | 35.9 | 24.5 | 32.6 | 32.3 | 26.1 |
| WCC (103/µL) | 16.4 | 11.3 | 6.8 | 7.9 | 6.6 | 6.6 | 4.8 | 8.3 |
| PMN (%) | 88 | 80 | 60 | 77 | 76 | 75.4 | ND | ND |
| Lymphocytes (%) | 4 | 8 | 23 | 14.7 | 14 | 16.5 | ND | ND |
| Thrombocytes (103/µL) | 232 | 251 | 171 | 180 | 177 | 215 | 23 | 370 |
| Urea (mg/dL) | 52 | 52 | 40 | 32 | 56 | 44 | 50 | 21 |
| Creatinine (mg/dL) | 1.3 | 1.2 | 1.3 | 1.2 | 1.8 | 1.6 | 1.3 | 0.9 |
| LDH (U/L) | 305 | ND | ND | ND | 263 | 223 | 229 | 259 |
| Haptoglobin (mg/dL) | 193 | ND | ND | ND | 10.8 | <5 | ND | ND |
| γ-GT (U/L) | 175 | ND | ND | ND | 303.8 | 236 | 304.8 | ND |
| GOT (U/L) | 24 | ND | ND | ND | 32 | 28 | 40 | ND |
| GPT (U/L) | 23 | ND | ND | 16 | 24 | 17 | 21 | ND |
| CRP (mg/L) | 159 | 87 | 31 | 26 | 9 | 6 | 8 | 38 |
The most important laboratory findings on the first admission were elevated WCC, a left-shift towards PMN and substantially raised CRP. All these parameters had considerably improved at the time of the first discharge and when the patient was seen in our outpatient clinic. The second admission was due to intolerance of TMP-SMX and haemolytic anaemia, while indicators of infection were low. The patient's third admission was for treatment of a newly diagnosed HCC and shows thrombocytopenia and worsening of anaemia due to linezolid treatment.
Normal values: RBC (4.2–6.3 106/µL), haemoglobin (12–18 g/dL), haematocrit (37–52%), WCC (4.3–10.0×103/µL), PMN (40–75%), lymphocytes (19–51%), urea (10–47 mg/dL), creatinine (0.5–1.1 mg/dL), LDH (135–225 U/L), haptoglobin (30–200 mg/dL), γ-GT (<66 U/L), GOT (10–50 U/L), GPT (10–50 U/L) and CRP <5 mg/L).
CRP, C reactive protein; GOT, glutamate oxaloacetate transaminase; GPT, glutamate-pyruvate transaminase; γ-GT,γ-glutamyltransferase; HCC, hepatocellular carcinoma; LDH, lactate dehydrogenase; ND, not determined; PMN, polymorphonuclear leucocytes; RBC, red blood cell; TMP-SMX, trimethoprim/sulfamethoxazole; WCC, white cell count.
Figure 1.
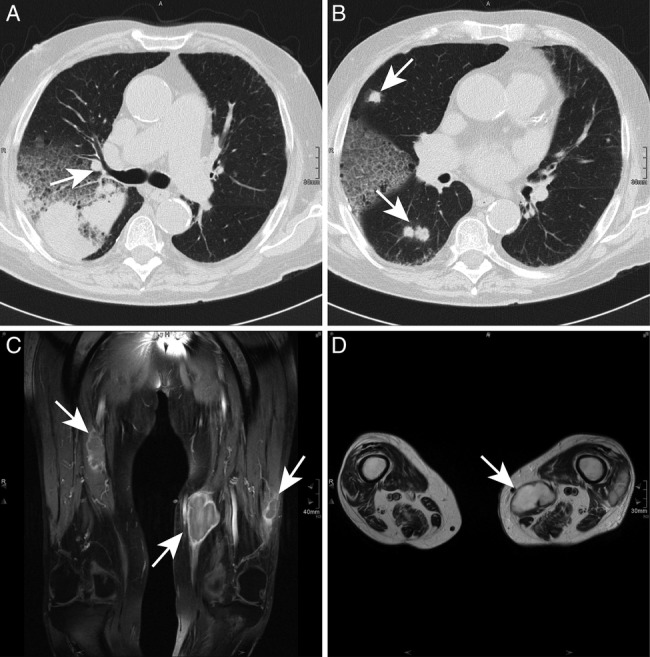
Chest CT (A and B) and MRI of both legs (C and D). Chest CT scan showing atelectasis of the dorsoapical segment of the right upper lobe and multiple round abscesses (white arrows) in both lungs. In the MRI, three intramuscular abscesses (white arrows) in the left sartorius muscle, left vastus lateralis muscle and right vastus medialis muscle are shown in a coronary contrast-enhanced T1 sequence and the two abscesses on the left side in a transversal T2 image.
A conventional MRI of both legs showed three lesions with hyperperfused rims, an intermediary to hypointense signal in T1 phases and a hyperintense T2 signal in the respective muscles (figure 1C, D). A needle aspiration was performed on the largest of the three lesions, which yielded very dense, yellow-brown pus. Gram stain showed Gram-positive, partially acid-fast branching rods. A CT of the brain and MRI of the abdomen were performed to rule out further abscesses.
Treatment
Empiric antibiotic therapy with clarithromycin and ceftriaxone for suspected community-acquired pneumonia was started initially. After the thoracic CT scan, metronidazole was added to the antibiotic regimen to cover bacteria found in pulmonary abscesses and the patient's immunosuppressive therapy was reduced to 5 mg prednisone daily. On the basis of the histology obtained during bronchoscopy, fluconazole was added to his medication. After needle aspiration of the leg abscess, the antibiotic regimen was changed to imipenem 4×500 mg/day, and the intramuscular abscesses were drained. The patient continuously improved and laboratory inflammatory markers were declining.
Outcome and follow-up
Subsequent culture of the aspirate yielded the subspecies N. otitidis-cavarium. After 21 days of intravenous treatment with imipenem, the patient was discharged on oral linezolid 2×600 mg/day. His immunosuppressive medication was resumed at a lower dose after his proteinuria had slightly risen to 350 mg/day (MMF 2×250 mg/day+prednisone 5 mg/day).
He was seen 1 week later in good health in our outpatient clinic. Laboratory parameters for infection were further declining. Meanwhile, susceptibility testing by E-test had shown sensitivity of the isolate to trimethoprim/sulfamethoxazole (TMP-SMX). His medication was switched to high-dose oral TMP-SMX (2×1920 mg/day). After 1 week, he was seen again, this time with nausea, vomiting, reduced appetite and general weakness. He was hospitalised and his laboratory findings showed a haemolytic anaemia, while infection parameters were unchanged. After other possible causes for haemolytic anaemia were ruled out, these symptoms were considered most likely a side effect of high-dose TMP-SMX. TMP-SMX was stopped and the patient was again given imipenem intravenously, resulting in prompt resolution of his symptoms.
During this stay, an abdominal ultrasound showed a suspicious hypoechogenic lesion in segment II/III of the liver. A liver biopsy was performed, which histologically showed hepatocellular carcinoma (HCC). While awaiting treatment for his HCC, he was discharged on oral linezolid 2×600 mg/day. His immunosuppressive medication was tapered as his membranous glomerulopathy was no longer regarded as idiopathic, but rather as tumour associated.
When the patient presented again for treatment of his HCC 2 weeks later, he was anaemic (haemoglobin 8.2 g/dL) and thrombocytopenic (24000/µL), which was attributed to linezolid after other possible causes had been ruled out. The patient was given imipenem intravenously and his blood values slowly recovered. After treatment of his HCC with radiofrequency ablation, he again developed subfebrile temperatures, and showed an increase in sputum production. Bronchoscopy and a CT scan were performed, which suggested an acute chronic obstructive pulmonary disease exacerbation rather than recurrence of his nocardial infection. Hence medication was switched from imipenem to levofloxacin, which had, meanwhile, also been shown to act effectively against his nocardial isolate. His clinical status improved further and he was discharged on oral levofloxacin as long-term therapy.
After discharge, the patient remained afebrile and his inflammatory parameters were further declining. Three weeks later, the patient died at home, presumably of an acute cardiac event.
Discussion
Our case highlights the variable clinical presentation of nocardial infection, which can mimic a whole variety of other clinical entities and can mislead even experienced clinicians.1 Especially in patients with comorbidities, a nocardial infection can be the common denominator of some seemingly distinct physical symptoms. Initially, our patient was thought to have a community-acquired pneumonia with coexisting peripheral arterial disease. After obtaining a chest CT scan, a metastasised bronchial adenocarcinoma was suspected, until bronchoscopy did not reveal a tumour but showed signs of severe acute infection. A conventional MRI of the legs did not support the notion of hypervascularised tumours, but rather pointed towards an abscess or infected haematoma. Only after aspiration of pus from one of three leg lesions and subsequent Gram and Ziehl-Neelsen staining as well as culture of the aspirate, could the diagnosis of systemic nocardiosis be established, 8 days after the patient was admitted to hospital.
Although we did not succeed in cultivating Nocardia from sputum samples obtained on the day of admission, nor from the bronchoalveolar lavage (BAL) specimens, the characteristic polymorphonuclear inflammation present in the bronchial cytology sample as well as the histological appearance of the transbronchial biopsy strongly suggest that Nocardia was the causative organism. In addition, location of pneumonia in the right upper field and the disseminated nature of abscesses throughout both lung lobes are suggestive of nocardial infection.7 It is likely that the lungs were the primary site of infection in our patient, as we could not delineate a causative trauma for a primary cutaneous infection. The skin covering the three abscesses was intact, and there were no additional signs of inflammation such as erythema or overwarmth. On the basis of ultrasound and MRI, all abscesses were intramuscular and approximately 3–5 cm beneath the skin.
In more than two-thirds of cases, the lungs are the primary site of Nocardia infection.4 It is thought that only about 10% of pulmonary infections disseminate to the skin, compared with 33%, which spread to the central nervous system.8 Intramuscular involvement seems to be very rare among all nocardial spp, in fact, we could only find five other published cases describing intramuscular abscesses.9 The initial histological evaluation of the transbronchial biopsy suggesting yeast as the causative infectious agent was misleading. In BAL and sputum specimens, there were only low numbers of Candida present (BAL: 103 fungi/mL), which points to an airway colonisation rather than a true Candida infection.
TMP-SMX is still the first-line therapy against nocardial infection, but over the last decade, an increased resistance was noted and other possible therapies emerged.4 Carbapenem antibiotics plus amikacin are a choice in severely ill patients until resistance testing is available.10 11 Unfortunately, resistance testing is hampered by the fact that there is no universally accepted method for Nocardia isolates. Although the Clinical and Laboratory Standards Institute (formerly NCCLS), in 2003, issued a recommendation that favoured broth microdilution over various other methods such as disc diffusion, agar dilution and E-test, these have been reported to give clinically relevant and useful results.3 10 11 Since of the severe systemic nocardial infection, we started to treat our patient with intravenous imipenem omitting amikacin due to his renal impairment.
All nocardial strains tested so far showed in vitro sensitivity to linezolid, and several reports suggest it as a new second-line agent for nocardial infections.12 Our patient showed clinical and laboratory improvement while taking linezolid for the first week, and also after his second hospital stay due to intolerance of TMP-SMX. However, after having taken linezolid for a total of 3 weeks, he developed severe haematological side effects with severe anaemia and thrombocytopenia, which necessitated changing his antibiotic therapy. These side effects seem to be limiting factors for long-term linezolid treatment of nocardial infection.2 We finally decided to use levofloxacin, which was both well tolerated and proved efficacious against this nocardial strain. On the basis of recent reports, fluoroquinolones should only be used after resistance testing has become available, because resistance can be as high as 50%.4 If fluoroquinolones are found to be active against Nocardia, they are a good treatment option due to their excellent oral bioavailability, good long-term tolerance and their tissue-penetrating characteristics.
Learning points.
Immunosuppressive medication should only be used very cautiously in elderly frail patients due to an increased risk of opportunistic infections.
Nocardial infections have a broad and highly variable clinical presentation. We describe the first patient with intramuscular abscesses due to Nocardia otitidis-cavarium.
Rapid and reliable resistance testing methods are currently lacking for nocardial isolates.
Long-term use of linezolid is limited by haematological side effects despite good in vitro susceptibility of Nocardia spp.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev 1994;7:213–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine 2004;83:300–13. 10.1097/01.md.0000141100.30871.39 [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliott BA, Brown JM, Conville PS et al. . Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 2006;19:259–82. 10.1128/CMR.19.2.259-282.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matulionyte R, Rohner P, Uckay I et al. . Secular trends of nocardia infection over 15 years in a tertiary care hospital. J Clin Pathol 2004;57:807–12. 10.1136/jcp.2004.016923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodfellow M, Lechevalier MP. Genus Nocardia Trevisan. In: Holt JG, Williams ST, Sharpe ME, eds. Bergey's manual of systematic bacteriology. Vol 4 Baltimore: Williams & Wilkins, 1989:2350–61. [Google Scholar]
- 6.Hachisuka H, Ichiki M, Yoshida N et al. . Primary subcutaneous abscess caused by Nocardia otitidiscaviarum. J Am Acad Dermatol 1989;21:201–11. 10.1016/S0190-9622(89)80355-X [DOI] [PubMed] [Google Scholar]
- 7.Feigin DS. Nocardiosis of the lung: chest radiographic findings in 21 cases. Radiology 1986;159:9–14. 10.1148/radiology.159.1.3952335 [DOI] [PubMed] [Google Scholar]
- 8.Yildiz O, Doganay M. Actinomycoses and Nocardia pulmonary infections. Curr Opin in Pulm Med 2006;12:228–34. 10.1097/01.mcp.0000219273.57933.48 [DOI] [PubMed] [Google Scholar]
- 9.Rees W, Schueler S, Hummel M et al. . Primary cutaneous Nocardia farcinica infection after heart transplantation: a case report. J Thorac Cardiovasc Surg 1995;109:181–3. 10.1016/S0022-5223(95)70436-1 [DOI] [PubMed] [Google Scholar]
- 10.Yildiz O, Alp E, Tokgoz B. Nocardiosis in a teaching hospital in the Central Anatolia region of Turkey: treatment and outcome. Clin Microbiol Infect 2005;11:495–9. 10.1111/j.1469-0691.2005.01145.x [DOI] [PubMed] [Google Scholar]
- 11.Lerner PI, Baum GL. Antimicrobial susceptibility of Nocardia species. Antimicrob Agents Chemother 1973;4:85–93. 10.1128/AAC.4.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moylett EH, Pacheco SE, Brown-Elliott BA et al. . Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis 2003;36:313–18. 10.1086/345907 [DOI] [PubMed] [Google Scholar]


