Figure 3. Closed and open conformations of the dimer of wild type protease and highly resistant mutant PR20.
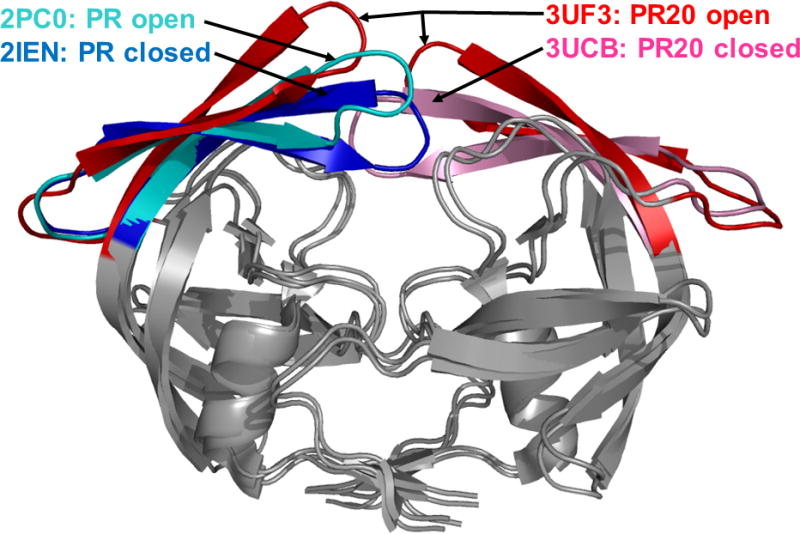
Superposition of the crystal structures of PR20 and wild type HIV protease. The dimers are shown in gray, except for the flaps, which are in different colors. The PDB ID is indicated for each structure. The different flap conformations highlight the variation observed in the crystal structures. The two closed conformation dimers are complexed with DRV and are similar in overall structure. The open conformation dimers contain no inhibitor. Open PR20 is shown with the two flaps in the dimer to indicate its atypical flap asymmetry, and the other structures are represented as single flaps.
For color images please see online www.future-science.com/doi/full/10.4155/FMC.15.44
