Figure 5. Darunavir and selected investigational inhibitors.
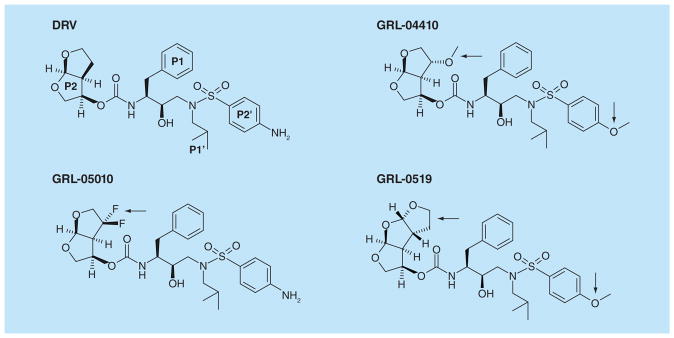
Structures are illustrated in similar conformations to facilitate comparison. Darunavir is shown with the P2, P1, P1’ and P2’ groups labeled. The modifications in the investigational inhibitors are indicated by black arrows. Antiviral inhibitors GRL-04410 and GRL-0519 have a methoxy substitution at P2’, while GRL-0519, GRL-04410 and GRL-05010 have tris-THF, methoxy and gem-difluoro additions at P2, respectively.
