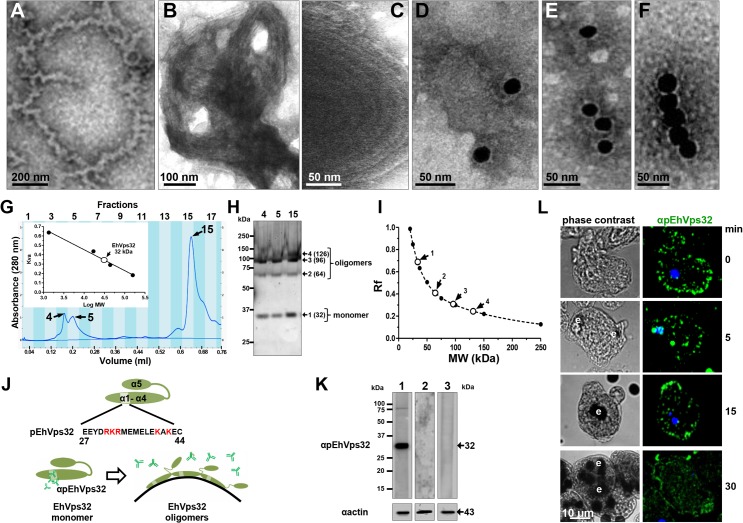
Fig 6. In vitro oligomerization of rEhVps32 and cellular localization of EhVps32 monomers.
(A-C) TEM of purified rEhVps32 (without GST-tag) negatively stained. (D-F) Samples were treated with αrEhVps32 antibodies, followed by gold-labeled secondary antibodies. (G) Gel filtration chromatographic profiles of purified rEhvps32 (without GST-tag). Inset: calibration curve using molecular weight standards to estimate the profile of EhVps32 monomer. (H) Eluted fractions contained the EhVps32 profiles indicated in the graph (G) were submitted to western blot analysis, using αrEhVps32 antibodies. (I). Rf of molecular weight markers (●) and bands (○) revealed by αrEhVps32 antibodies in (H). (J) Schematic representation of close and open conformations of EhVps32 showing the position of pEhVps32 polypeptide used to generate αpEhVps32 antibodies. Red letters: positively charged residues. Scheme shows the EhVps32 monomers in close conformation and the EhVps32 oligomers in open conformation on the phagosome membrane. (K) Western blot assays of trophozoites lysates, using αpEhVps32 antibodies alone (lane 1), competed with rEhVps32 purified protein (lane 2) or competed with αrEhVps32 antibodies (lane 3). As a loading control, the same membranes were re-blotted with αactin antibodies. (L) Confocal microscopy of trophozoites incubated with erythrocytes during different times, treated with αpEhVps32 and FITC-labeled secondary antibodies. Nuclei were counterstained with DAPI. e, erythrocytes.