Abstract
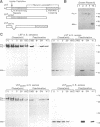
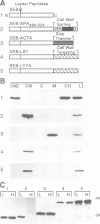
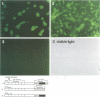
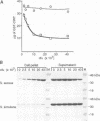
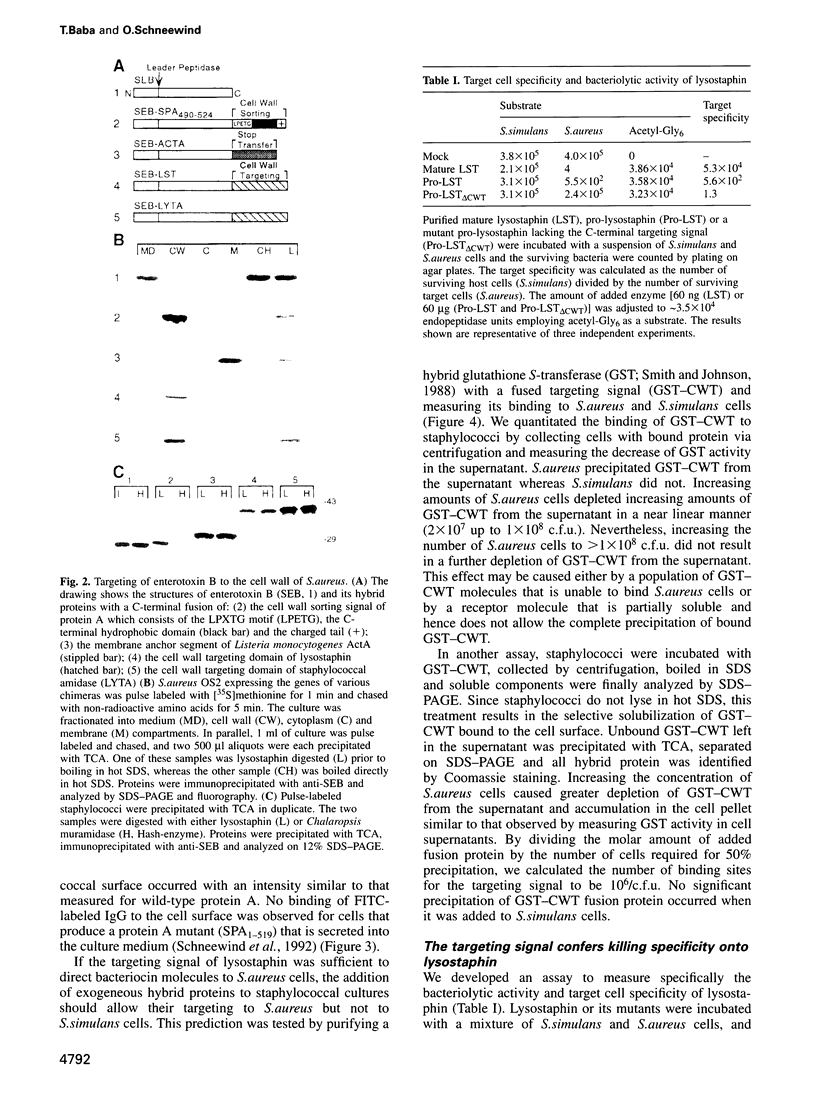
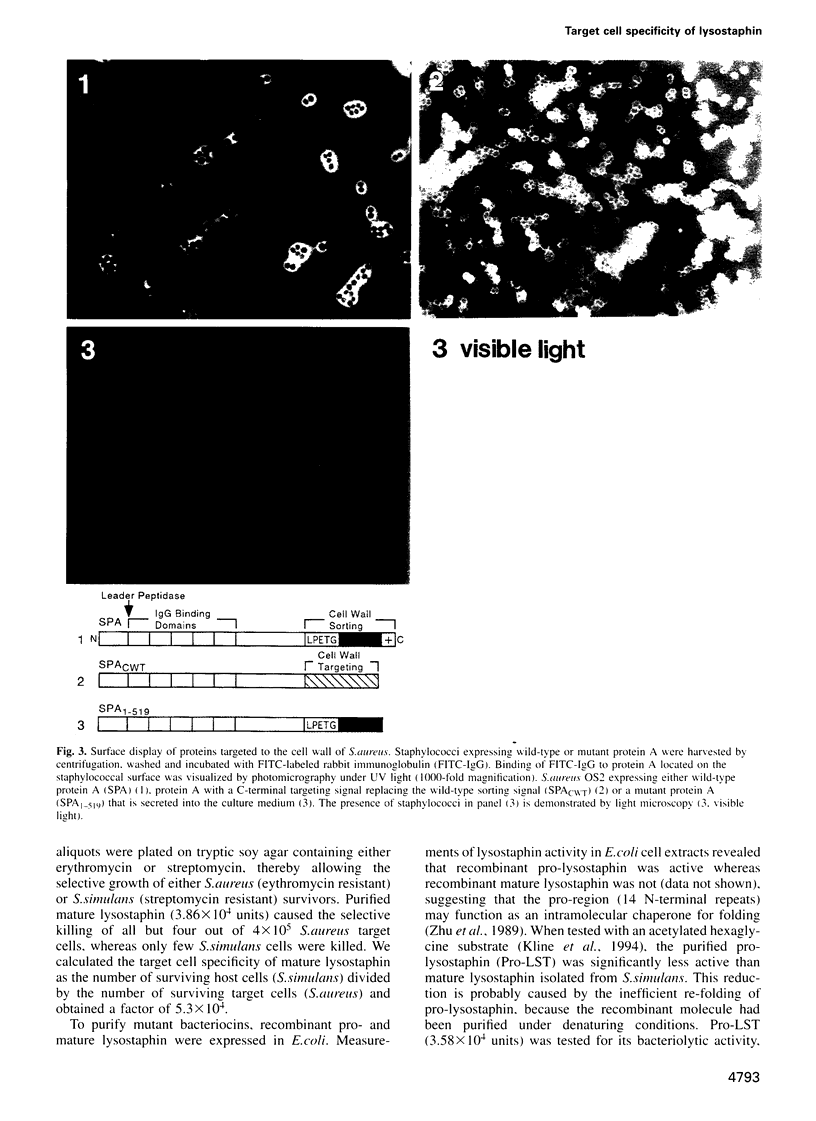
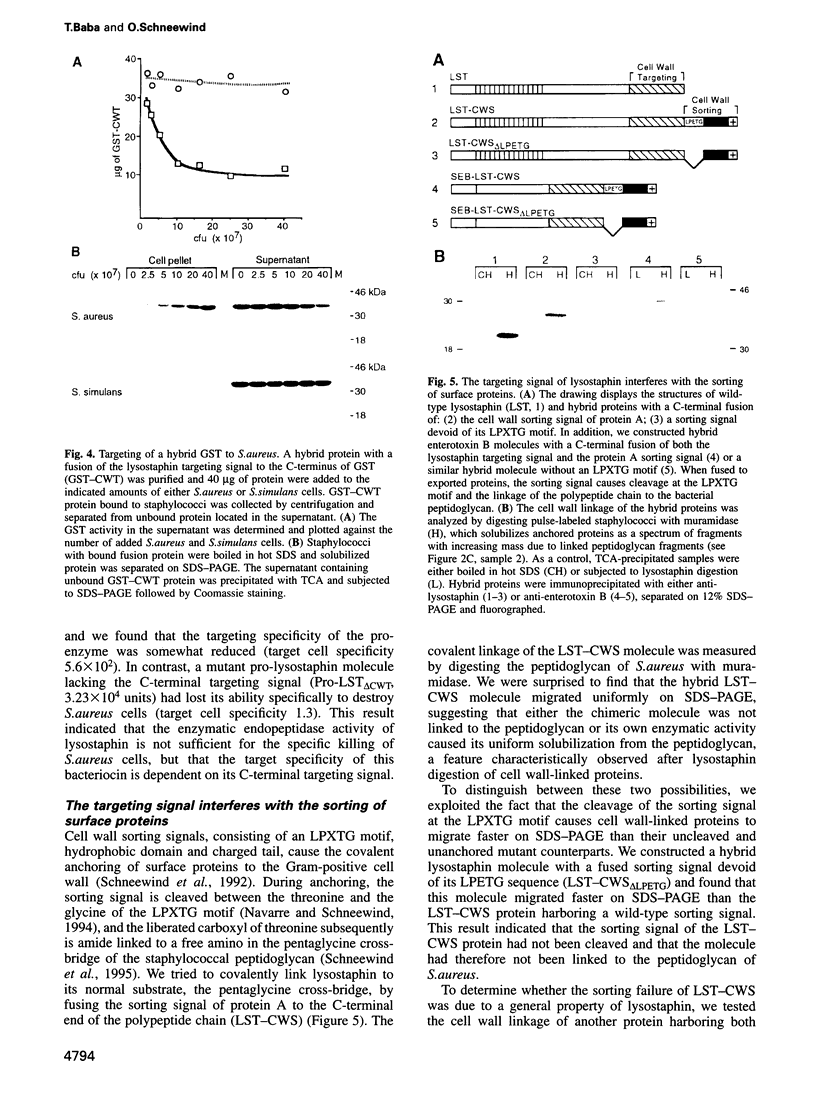
Microbial organisms secrete antibiotics that cause the selective destruction of specific target cells. Although the mode of action is known for many antibiotics, the mechanisms by which these molecules are directed specifically to their target cells hitherto have not been described. Staphylococcus simulans secretes lysostaphin, a bacteriolytic enzyme that cleaves staphylococcal peptidoglycans in general but that is directed specifically to Staphylococcus aureus target cells. The sequence element sufficient for the binding of the bacteriocin as well as of hybrid indicator proteins to the cell wall of S.aureus consisted of 92 C-terminal lysostaphin residues. Targeting to the cell wall of S.aureus occurred either when the hybrid indicator molecules were added externally to the bacteria or when they were synthesized and exported from their cytoplasm by an N-terminal leader peptide. A lysostaphin molecule lacking the C-terminal targeting signal was enzymatically active but had lost its ability to distinguish between S.aureus and S.simulans cells, indicating that this domain functions to confer target cell specificity to the bacteriolytic molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Benedetti H., Frenette M., Baty D., Knibiehler M., Pattus F., Lazdunski C. Individual domains of colicins confer specificity in colicin uptake, in pore-properties and in immunity requirement. J Mol Biol. 1991 Feb 5;217(3):429–439. doi: 10.1016/0022-2836(91)90747-t. [DOI] [PubMed] [Google Scholar]
- DeHart H. P., Heath H. E., Heath L. S., LeBlanc P. A., Sloan G. L. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl Environ Microbiol. 1995 Apr;61(4):1475–1479. doi: 10.1128/aem.61.4.1475-1479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E., García E., Ascaso C., Méndez E., López R., García J. L. Subcellular localization of the major pneumococcal autolysin: a peculiar mechanism of secretion in Escherichia coli. J Biol Chem. 1989 Jan 15;264(2):1238–1244. [PubMed] [Google Scholar]
- Garrido M. C., Herrero M., Kolter R., Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988 Jun;7(6):1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- Heath H. E., Heath L. S., Nitterauer J. D., Rose K. E., Sloan G. L. Plasmid-encoded lysostaphin endopeptidase resistance of Staphylococcus simulans biovar staphylolyticus. Biochem Biophys Res Commun. 1989 May 15;160(3):1106–1109. doi: 10.1016/s0006-291x(89)80117-2. [DOI] [PubMed] [Google Scholar]
- Heinrich P., Rosenstein R., Böhmer M., Sonner P., Götz F. The molecular organization of the lysostaphin gene and its sequences repeated in tandem. Mol Gen Genet. 1987 Oct;209(3):563–569. doi: 10.1007/BF00331163. [DOI] [PubMed] [Google Scholar]
- Jack R. W., Tagg J. R., Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995 Jun;59(2):171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline S. A., de la Harpe J., Blackburn P. A colorimetric microtiter plate assay for lysostaphin using a hexaglycine substrate. Anal Biochem. 1994 Mar;217(2):329–331. doi: 10.1006/abio.1994.1127. [DOI] [PubMed] [Google Scholar]
- Kolter R., Moreno F. Genetics of ribosomally synthesized peptide antibiotics. Annu Rev Microbiol. 1992;46:141–163. doi: 10.1146/annurev.mi.46.100192.001041. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre W. W., Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994 Oct;14(1):115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- Oshida T., Sugai M., Komatsuzawa H., Hong Y. M., Suginaka H., Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattus F., Massotte D., Wilmsen H. U., Lakey J., Tsernoglou D., Tucker A., Parker M. W. Colicins: prokaryotic killer-pores. Experientia. 1990 Feb 15;46(2):180–192. [PubMed] [Google Scholar]
- Pilsl H., Braun V. Evidence that the immunity protein inactivates colicin 5 immediately prior to the formation of the transmembrane channel. J Bacteriol. 1995 Dec;177(23):6966–6972. doi: 10.1128/jb.177.23.6966-6972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P. A., Gruss A. D., Novick R. P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1127–1131. doi: 10.1073/pnas.84.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J. M., Díaz E., García J. L. Studies on the structure and function of the N-terminal domain of the pneumococcal murein hydrolases. Mol Microbiol. 1992 Apr;6(7):921–931. doi: 10.1111/j.1365-2958.1992.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Fowler A., Faull K. F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995 Apr 7;268(5207):103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Mihaylova-Petkov D., Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993 Dec;12(12):4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V. A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992 Jul 24;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Sloan G. L., Smith E. C., Lancaster J. H. Lysostaphin endopeptidase-catalysed transpeptidation reactions of the imino-transfer type. Biochem J. 1977 Oct 1;167(1):293–296. doi: 10.1042/bj1670293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Iandolo J. J. Transport and processing of staphylococcal enterotoxin B. J Bacteriol. 1983 Jan;153(1):297–303. doi: 10.1128/jb.153.1.297-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Z., Projan S. J., Novick R. P. Nucleotide sequence of beta-lactamase regulatory genes from staphylococcal plasmid pI258. Nucleic Acids Res. 1991 Jul 25;19(14):4000–4000. doi: 10.1093/nar/19.14.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wilkinson B. J., Jayaswal R. K. Sequence analysis of a Staphylococcus aureus gene encoding a peptidoglycan hydrolase activity. Gene. 1991 Jun 15;102(1):105–109. doi: 10.1016/0378-1119(91)90547-o. [DOI] [PubMed] [Google Scholar]
- Yamada S., Sugai M., Komatsuzawa H., Nakashima S., Oshida T., Matsumoto A., Suginaka H. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996 Mar;178(6):1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989 Jun 8;339(6224):483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]